Abstract
Bacterial infection is one of the major causes of morbidity and mortality especially in developing countries. The aim of this study was to develop a new radiopharmaceutical for imaging infection. The labeling conditions were optimized, and lyophilized kits were developed for instant preparing. The stability of 99mTc-AMOX in human serum was identified, sterility and pyrogenicity of the radiopharmaceutical were estimated, gamma scintigraphy and in vivo biodistribution with infected rats were investigated. The promising properties of 99mTc-AMOX combined with the development of reliable and instant lyophilized kit afford the opportunity of inflammatory process imaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, acute, subacute and chronic bacterial infections are major causes of mortality and morbidity [1]. The in-time diagnosis of bacterial infection is extremely significant for the accurate treatment [2]. Clinicians use variety of physical examinations, laboratory and radiological tests to aid the diagnosis and make decisions [1]. Although the radiological imaging techniques including computed tomography (CT), nuclear magnetic resonance (NMR) and ultrasonography (US) are commonly used, these techniques are limited use due to insignificant anatomical changes in early stage of the disease. In patients with serious underlying conditions, the identification of infection at early stage of the disease is critical for a favorable outcome [1–4].
Infection detection by nuclear medicine imaging techniques based on pathophysiological and pathobiological changes which appear much earlier than anatomical changes in the infection process [5, 6]. Nuclear medicine techniques require reliable radiopharmaceuticals that can selectively concentrate in the site of infection [7]. 67Ga-citrate and 111In-labeled leucocytes have shown promising diagnostics results but awkward methods of formulation and high radiation dose limit their use in clinics [2]. Since the favorable properties such as 140 keV pure gamma energy and the half-life of 6 h, 99mTc is the most widely used radionuclide for diagnostic applications in nuclear medicine departments. The use of 99mTc labeled antibiotics for infection imaging is promising because of their specific activity against bacteria. In recent years 99mTc labeled ciprofloxacin (infecton) were evaluated as an infection imaging agent. [8, 9]. Two vial cold kits of infecton which was further modified to a single vial, has some bottlenecks in the use of the kit such as need for filtration during reconstitution caused additional radiation burden and cause the loss of radioactivity [5, 9–11].
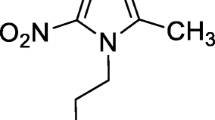
To develop a better infection imaging which will accumulate efficiently to inflammatory foci, clear rapidly from background tissue, discriminate between bacterial infection and sterile inflammation, cost low and prepare easily we labeled amoxicillin sodium (AMOX) with 99mTc. AMOX is a semisynthetic antibiotic and is a member of the penicillinase-stable group of penicillins derived from the penicillin nucleus (Fig. 1) [12].
In this study, we prepare 99mTc-AMOX in a simple radiochemical method with good labeling efficiency and evaluate the ready to use cold kit formulation thus making it available to the other nuclear medicine centers. The stability of 99mTc-AMOX in human serum was identified, sterility and pyrogenicity of the radiopharmaceutic were estimated and gamma scintigraphy studies with bacterial infected and sterile inflamed rats were investigated.
Materials and methods
Materials
All chemicals and solvents were used without further purification. AMOX was a gift from AppliChem, (Germany). Stannous tartrate and stannous chloride dehydrate were purchased from Sigma-Aldrich (USA) and ascorbic acid was purchased from Sigma-Aldrich (United Kingdom). 99mTc was eluted from the 99Mo (99Mo)/99mTc-generator (Nuclear Medicine Department of Ege University). Thioglycollate Broth (TB) and Tryptic Soy Broth (TSB) mediums and all solvents were obtained from Merck (Germany). Dose Calibrator (Atomlab 100, Biodex Medical Systems) was used for counting radioactive samples. Escherichia coli (ATCC 25922) bacteria were obtained from Pharmaceutical Microbiology Department of Ege University. The Animal Ethics Committee of the Ege University gave approval for the animal experiments (Number: 2010-37, 2010). Results are reported as mean ± standard error.
Radiolabeling studies
To investigate the optimum radiolabeling conditions, radiolabeling was tested with different concentrations of reducing (stannous chloride and stannous tartrate) and antioxidant (ascorbic acid) agent. Radiochemical analysis was performed with Radio Thin Layer Chromatography (RTLC) and Radio High Performance Liquid Chromatography (RHPLC) studies. Two different freeze dry kits were formulated with optimum labeling conditions and stability of the kits were performed.
Effect of reducing agent on labeling
Two different concentrations of AMOX solution were prepared in saline. To lower concentrated stock solution (5 mg/1 mL), stannous chloride was added under an atmosphere of bubbling nitrogen. Reduction of 99mTc was performed with different amount of stannous chloride in 0.1 N HCl (30, 80, 100, 150 and 200 µg). To higher concentrated stock solution (10 mg/1 mL), different amount of stannous chloride in 0.1 N HCl (100, 150 and 200 µg) was added under an atmosphere of bubbling nitrogen. Radiolabeling was performed with 99mTc (37 MBq) in saline (0.1 mL) and solution was allowed to stand at room temperature for 15 min prior to radiochemical analyses.
Reducing agent effect on labeling efficiency was also performed with the formulations described above by using stannous tartrate instead of stannous chloride.
Effect of antioxidant agent on labeling
To examine the effect of antioxidant agent on labeling efficiency and stability of the complex, labeling studies were performed in the absence and presence of antioxidant agent. AMOX solution were prepared (10 mg/1 mL), in three groups of vials. 100, 150 and 200 µg stannous chloride was added to each individual group. Each group has three vials and labeling was performed in the absence and presence (1.5 and 3.0 mg) of ascorbic acid. Freshly eluted 37 MBq 99mTc was added to each vial. The vials were shaken for 30 s, filtered through a 0.22 µm pore size filter and incubated for 15 min at room temperature. The labeling efficiency was analyzed by RTLC.
Antioxidant agent effect on labeling efficiency was also performed with ideal formulation by using stannous tartrate instead of stannous chloride.
Effect of incubation time on labeling
After radiolabeling the radiochemical purity of the complexes were investigated with RTLC studies which performed at different time intervals (5, 15, 30, 45 and 60 min postlabeling).
Effect of pH on labeling
The radiochemical purity of a 99mTc radiopharmaceutical is highly dependent on the pH of the kit mixture. To investigate the effect of the pH on labeling efficiency, 99mTc-AMOX solution pH was adjusted to 4.8–7.4 by adding 0.1 N NaOH.
Stability of 99mTc-AMOX in saline
100 µL of 99mTc-AMOX reaction medium was added to 1 mL of saline. The mixture was incubated at 37 °C for 24 h. Two drops of sample was spotted on chromatographic strips at different time intervals up to 24 h and RTLC studies were performed to determine the stability of 99mTc-AMOX in saline.
Stability of 99mTc-AMOX in Human Serum
100 µL of 99mTc-AMOX reaction medium was added to 1 mL of freshly prepared human serum. The mixture was incubated at 37 °C for 24 h. Two drops of sample was spotted on chromatographic strips at different time intervals up to 24 h and RTLC studies were performed to determine the stability of 99mTc-AMOX in human serum
RTLC procedure
Silica gel coated fiber sheets (SG) and instant thin layer silica gel papers (ITLC-SG) were used as stationary phase. Free and Reduced/Hydrolyzed (R/H) 99mTc were determined by using methyl ethyl ketone (MEK) and butanone/ethanol/water (B/E/W) (35/35/30) as the mobile phases respectively. The radioactivity on plates was measured using a TLC scanner (Bioscan AR 2000), and % radiochemical purity (RP) of 99mTc-AMOX was calculated from the following equation by subtracting from 100 the sum of measured impurities percentages.
RHPLC analysis
The compounds were further analyzed by an Ultra-HPLC system equipped with a C18 column connected to a photodiode array detector (PDA) and additional NaI gamma detector for the 99mTc compounds. The flow rate was 1.0 mL/min for analytical runs. In all runs the eluent was 0.1 % TFA in H2O (solvent A) and 0.1 % TFA in ACN (solvent B). For the analytical control and semi preparative separation the method was as follows: 0–2.5 min 95 % solvent A-5 % solvent B; 2.5–5 min 50 % solvent A 50 %-solvent B; 5–7.5 min 20 % solvent A 80 %-solvent B; 7.5–10 min 5 % solvent A-95 % solvent B.
Freeze dry kit formulation and radiolabeling
After observing the effect of different parameters on labeling, subsequently two lots were prepared as follows: Lot A was prepared by mixing 10 mg AMOX, 200 µg stannous tartrate and 3 mg ascorbic acid. Lot B was prepared by mixing 10 mg AMOX, 200 µg stannous chloride and 3 mg ascorbic acid. Both kits solution were filtered through a 0.22 µm pore size filter to glass vials, filtered solutions were frozen in a freezer at −80 °C and lyophilized at −20 °C for 24 h.
In vitro radiolabeling studies were performed with 37 MBq 99mTc for the regard to radiation safety of personnel and the environment. Freeze dry were labeled with 37–370 MBq 99mTc.
Stability of the freeze dry kits
The kits were stored at +5 ± 3 °C (in a refrigerator) and +25 °C ± 2 °C/60 %RH (relative humidity) ± 5 % (in a stability cabin). The labeling efficiency of 99mTc-AMOX in each kit was checked at different time intervals up to 6 months. The kits were reconstituted with 37 MBq of 99mTc-pertechnetate and the radiochemical purity was determined by RTLC and RHPLC studies.
Microbiological analysis of 99mTc-AMOX Kit
Sterility test
The sterility of the kits was tested by direct inoculation method according to the European Pharmacopeae 6.0 recommendations. The dilutions of the kits were aseptically inoculated to the sterilized TB and TSB medium vials, and incubated at 35 ± 2 °C. At the end of the incubation, absence of clearly visible growth of microorganisms in the vials exhibited the sterility.
Pyrogenicity test
Pyrogenicity test was performed by the gel clot method which based upon the reaction between bacterial endotoxin and the specific lysate. In the presence of endotoxin the gel is formed via a clotting reaction and the sample is fail. Endotoxin limit value of the kit and Maximum Valid Dilution (MVD) was estimated according to European Pharmacopeia 6.0. The LAL test is performed by Pyrotell (Associates of Cape Cod, Incorporated, Falmouth, Massachusetts) Gel-Clot formulation.
Animal studies
Wistar albino rats (200–250 g) were used for animal studies. Experiments with rats were performed according to a protocol approved by Animal Ethics Committee of the Ege University.
Induction of bacterial infection model
E.coli suspension was used to create infection model. 200 µL (4 × 1010cfu/1 mL) E.coli suspension was intramuscularly injected into the right thigh muscle of rats (n = 6). Then the infection was allowed to develop for 24 h.
Induction of sterile inflammation model
200 µL Turpentine oil was intramuscularly injected into the left thigh muscle of rats (n = 6). Then the inflammation was allowed to develop for 24 h.
Scintigraphic imaging studies of 99mTc-AMOX
After the infection and sterile inflammation focus were allowed to develop for 24 h swelling appeared and gamma scintigraphy studies were performed. 99mTc-AMOX (3.7 MBq) was intravenously injected via tail vein to the rats. During the scintigraphy studies animals were under anesthetize with Ketamine/Xylazin cocktail. The scintigraphic images were obtained with a dual head gamma camera (Infinia General Electric) equipped with a low-energy high-resolution collimator viewing the whole body of rats. After administration of radiopharmaceuticals, serial static images were acquired in a 256 × 256 matrix for 60 s each, at different time intervals (5 min, 1, 2, 3, 4 and 5 h post injection).
Biodistribution studies
5 h after injection, the rats were sacrificed and biodistribution was determined. Samples of infected muscle, inflamed muscle, healthy muscle, blood, liver, spleen, lung, kidney, stomach, intestine, urine and heart were weighed and the radioactivity was measured using a gamma counter (Sesa Uniscaller). The results were expressed as the percentage of injected dose per gram of tissue (%ID/g).
Statistical analysis
The calculation of means and standard deviations were made on Microsoft Excel. Oneway Anova was used to determine statistical significance. Differences at the 95 % confidence level (p < 0.05) were considered significant.
Results and discussion
Radiolabeling studies
A new, simple, rapid and efficient direct method for labeling of AMOX with 99mTc was developed. Labeling efficiency of the 99mTc-AMOX was assessed by both RTLC and RHPLC studies. Two solvent systems were used to distinguish and quantify the amounts of radioactive contaminants.
In RTLC, using MEK as the solvent, free 99mTc moved with the solvent front, while 99mTc-AMOX and R/H 99mTc remained at the spotting point. R/H 99mTc was determined by using B/E/W (35/35/30) as the mobile phase where the R/H 99mTc remained at the point of spotting while free 99mTc and 99mTc-AMOX moved with the solvent front. The Rf values of 99mTc-AMOX in mobile phases was presented in Table 1.
The radiochemical purity of 99mTc-AMOX was found >95 %. The RHPLC chromatogram was presented in Fig. 2 and showed two peaks, first one was corresponds to free 99mTc, while the second peak for 99mTc-AMOX.
99mTc may be formed by interactions between electron donor atoms such as nitrogen, oxygen, sulfur and reduced technetium. Due to presence of electron donor atoms in amoxicillin structure the reduced sodium pertechnetate can easily react with this ligand and a complex is formed. To determine exact structure of the complex further research is necessary.
Effect of reducing agent on labeling
To investigate the effect of reducing agent type on labeling yield, labeling experiments were performed with the same amounts of active ingredient, reducing agent and radionuclide including formulations. Two groups of formulation were prepared. While first group include lower concentration of AMOX (5 mg/1 mL), second group include higher concentration of (10 mg/1 mL) instead. Comparative results for both formulations were shown in Figs. 3 and 4 respectively.
The effect of reducing agent concentration on the radiochemical purity was evaluated and optimum reducing agent amount found 200 µg. Under these conditions labeling efficiency was around 90 %.
According to the results there were no differences found between the labeling efficiency of same amounts of stannous tartrate or chloride included formulation. So two lots of freeze dry kit were prepared and reducing agent type on the stability o kits was studied.
Effect of antioxidant agent on labeling
In the presence of ascorbic acid, stability of the complex was slightly increase while labeling efficiency for early hours was not affected significantly. R/H 99mTc percentage was decreased in the presence of ascorbic acid. The results in these experiments revealed that, maximum radiochemical purity was obtained with 10 mg AMOX, 200 µg reducing agent (stannous chloride, stannous tartrate) and 3 mg ascorbic acid including formulations (Tables 2, 3, 4 and 5). Effect of incubation time, pH and stability of these formulations were investigated to evaluate the optimum labeling conditions for AMOX.
Effect of incubation time on labeling
200 µg stannous chloride or stannous tartrate and 3 mg ascorbic acid including formulations were labeled with 37 MBq 99mTc. The radiochemical purity of the complexes was investigated with RTLC studies at 5, 15, 30, 45 and 60 min postlabeling (Table 6). According to the experiments, the formation of the 99mTc-AMOX complex was very fast and reached the radiochemical purity over 95 % in 15 min after radiolabeling.
Effect of pH on labeling
The effect of pH on radiochemical purity was examined at pH 4.8-7.4. According to the experiments results the pH of the reaction medium was not found to play an important role in the labeling process (Fig. 5). While keeping other reaction conditions constant and varied the pH of the reaction from 4.8 to 7.4 radiochemical purity was slightly decreased.
Stability of 99mTc-AMOX in saline
99mTc-AMOX was found stable during to 24 h incubation in saline (Fig. 6).
Stability of 99mTc-AMOX in Human Serum
99mTc-AMOX was found stable during to 24 h incubation in human serum (Fig. 7).
Stability of the freeze dry kits
Kits were labeled with 37, 185 and 370 MBq 99mTc. Slightly decrease in radiochemical purity was observed with increasing of radioactivity (Fig. 8).
The stability of the freeze dry kits which stored in +5 ± 3 °C and +25 ± 2 °C/60 %RH ± 5 % was determined at different time intervals up to 6 months. According to experiments, both kits (Lot A and Lot B) were found stable up to 6 months without any significant decrease in radiochemical purity (Fig. 9).
The freeze dry kits developed in this study were found to be stable with a shelf–life of 6 months when preserved at both at +5 ± 3 °C and +25 ± 2 °C/60 %RH ± 5 %. Based on the findings of this study, by the reconstitution of these kits the complex easily formed without any requirement for boiling and post-labeling purification. Unfortunately there were no statistical differences found for labeling efficiency and stability of the different reducing agent included kits, 200 µg stannous tartrate included formulation was found optimal since to have higher labeling yield.
Microbiological analysis of 99mTc-AMOX Kit
According to sterility test, since there was no growth in batches, kits were sterile. Also gel clot test showed that kits were pyrogen free.
Scintigraphic imaging studies of 99mTc-AMOX
The uptake of 99mTc-AMOX following intravenous administrations was assessed on static images. The images depicted rapid distribution throughout the body and uptake in the bacterial infected and sterile inflamed thigh muscle within 1 hour after injection. As can be seen in Fig. 10 there was a higher activity in both bacterial infected and sterile inflamed thigh muscle as compared to healthy thigh muscle.
For quantitative evaluation, regions of interest were drawn around the target (infected and inflamed thigh muscle) and non-target (healthy thigh muscle) regions of the rats. The 99mTc-AMOX uptake was calculated by dividing the average counts per pixel within the region of target to the average counts per pixel within the region of non-target (Table 7). These ratios indicated a significant uptake of 99mTc-AMOX in infection and inflammation foci. The data suggest that 99mTc-AMOX remained at the infection and inflammation foci during the whole experiments, there being no statistically significant differences in the ratios during the studied period (p ≥ 0.05).
Biodistribution studies
Based on data presented in Tables 8 and 9, biodistribution of 99mTc-AMOX was determined for lung, kidney, spleen, intestine, heart, blood, liver stomach infected, inflamed and healthy thigh muscle. Moderate clearance from the kidney 1.34 ± 0.96–0.88 ± 0.13 %ID/g was observed. The presence of high activity in the kidney suggests the urinary system being the major route of excretion of the administered radiopharmaceutical. Amoxicillin has a mean elimination half-life of approximately 1 h and 60–70 % of the amoxicillin and is excreted unchanged in urine during the first 6 h after administration of a single 500/100 bolus intravenous injection. Various studies have found the urinary excretion to be 50-85 % for amoxicillin over a 24 h period [15]. So the authors can discuss the de-corporate 99mTc may be responsible for the gastrointestinal radioactivity 5 h after administration. The 99mTc-AMOX uptake was found 0.15 ± 0.13 %ID/g (Table 9), 0.07 ± 0.02 %ID/g for infected and inflamed thigh muscle of rats respectively.
Conclusion
AMOX, is s a member of the penicillinase-stable group of penicillins derived from the penicillin nucleus. So far various antibiotics were labeled with 99mTc. AMOX was previously labeled by Örümlü [13, 14]. The aim of this study was to standardize and develop a new, simple and ready to use kit of AMOX for radiolabeling with 99mTc. 99mTc-AMOX was developed and standardized under varying conditions of reducing and antioxidant agent concentration, pH, radioactivity dose and reducing agent type. Labeling studies were performed by changing the selected parameters one by one and optimum labeling conditions were determined. After observing the conditions for maximum labeling efficiency and stability, lyophilized freeze dry kits were prepared accordingly.
Simple method for radiolabeling of AMOX with 99mTc has been developed and standardized. Labeling efficiency of 99mTc-AMOX was assessed by both RTLC and RHPLC and found higher than 90 %. The resulting complex was quite stable and labeling of >90 % was maintained for up to 6 h. Two different freeze dry kits was developed and evaluated. Based on the data obtained from this study, both products was stable for 6 months with high labeling efficiency. To examine the role of 99mTc-AMOX in imaging of infection at early stage, in vivo studies are in progress.
The labeled compound was found to be stable in human serum up to 24 h. The prepared cold kit was found sterile and pyrogen free. The bacterial infection and sterile inflammation imaging capacity of 99mTc-AMOX was evaluated. Based on the in vivo studies, 99mTc-AMOX has higher uptake in infected and inflamed thigh muscle than healthy muscle. The data suggest that 99mTc-AMOX remained at the infection and inflammation foci during the whole experiments, there being no statistically significant differences in the ratios during the studied period (p ≥ 0.05).
References
El-Ghany EA, El-Kolaly T, Amine AM, El-Sayed AS, Abdel-Gelil F (2005) Synthesis of 99mTc-pefloxacin: a new targeting agent forinfectious foci. J Radioanal Nucl Chem 266:131–139
Das SS, Hall AV, Wareham DDW, Britton KE (2002) Infection imaging with radiopharmaceuticals in the 21st century. Braz Arch Biol Technol. 45:25–37
Shah SQ, Khanb MR (2012) Radiosynthesis of 99mTc-Labeled Novobiocin Dithiocarbamate Complex as a Potential Staphylococcus aureus Infection Radiotracer. Radiochemistry 54(3):279–283
Nibbering PH, Welling MM, Paulusma-Annema A, Brouwer CPJM, Lupetti A, Pauwels EKJ (2004) 99mTc-Labeled UBI 29-41 Peptide for Monitoring the Efficacy of Antibacterial Agents in Mice Infected with Staphylococcus aureus. J Nucl Med 45:321–326
Kaul A, Hazari PP, Rawat H, Singh B, Kalawat TC, Sharma S, Babbar AK, Mishra AK (2013) Preliminary evaluation of technetium-99m-labeled ceftriaxone: infection imaging agent for the clinical diagnosis of orthopedic infection. Int J Infect Dis. 17(4):e263–e270
Gemmel F, Dumarey N, Welling M (2009) Future diagnostic agents. Semin Nucl Med 39:11–26
Fazli A, Saluti M, Ahmadi Gh, Mirshojaei F, Mazidi M, Heydari Z (2012) Radiolabeling of ceftriaxone with 99mTc as a targeting radiopharmaceutical for staphylococcus aureus detection in mouse model. Iran J Med Phys 9(2):103–110
Britton KE, Wareham DW, Das SS, Solanki KK, Amaral H et al (2002) Imaging bacterial infection with 99mTc-ciprofloxacin (Infecton). J Clin Pathol 55(11):817–823
Britton KE, Vinjamuri S, Hall AV, Solanki K, Siraj QH, Bomanji J, Das S (1997) Clinical evaluation of technetium-99m infecton for the localisation of bacterial infection. Eur J Nucl Med 24(5):553–556
Pauwels EK, Welling MM, Lupetti A, Nibbering PH (2001) Concerns about 99mTc-labelled ciprofloxacin for infection detection—reply. Eur J Nucl Med 28:781
Signore A, D’Alessandria C, Lazzeri E, Dierck R (2008) Can we produce an image of bacteria with radiopharmaceuticals? Eur J Nucl Med Mol Imaging 35:1051–1055
Çağlayan Örümlü O (2010) Development new radiopharmaceutics for imaging acute infection and inflammation, (PhD Thesis), Ege University Radiopharmacy Department, İzmir
Örümlü O, Aşıkoğlu M (2004) Labelling od Amoxicillin Sodium with 99mTc’. In: The 4th international postgraduate research symposium on pharmaceuticals’, Istanbul. 20–22 Sept 2004
http://www.medicines.ie/medicine/2183/SPC/Augmentin+Intravenous+1.2g/
Acknowledgments
This study was supported by The Scientific and Technological Research Council of Turkey (Tubitak-110 S 229). The authors would like to acknowledge the support of T.R. Prime Ministry State Planning Organization Foundation Grant Project Number: 09DPT001 for TLC Scanner. Also the authors thank to Ege University Nuclear Medicine Department technicians for their technical assistance for the animal experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ilem-Ozdemir, D., Caglayan-Orumlu, O., Asikoglu, M. et al. Evaluation of 99mTc-amoxicillin sodium as an infection imaging agent in bacterially infected and sterile inflamed rats. J Radioanal Nucl Chem 308, 995–1004 (2016). https://doi.org/10.1007/s10967-015-4516-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4516-4