Abstract
Monitoring of radioactivity in an environment is an important step towards establishing the baseline for environment protection. This work highlights the spatial distribution of uranium (U); assessment of physico-chemical parameters and radiological assessment in groundwater of Hamirpur district, HP, India. The concentration of U ranges from 0.15 to 18.92 μg l−1. High correlation of U is observed with TDS, Chloride and Nitrate. Major concentration of U is present along the NW–SE trend-line following the Siwalik in the areas where mineralisation is prevalent and is of geogenic source derived from granitic and volcanic rock fragments present in conglomerates. The water-quality-index indicates that the water is suitable for drinking purpose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural radiation and radioactivity have always been a part of the environment. All people are exposed to natural radiations via food, water, air and soil. These radiations originate from a radioactive element like uranium and its daughter products like radon gas. The presence of natural radionuclide like uranium (U) in water is an area of concern these days. In recent years, many studies have reported the occurrence of U in drinking water [1,2,3,4]. U is the toxic and natural radioactive element having mainly 3 isotopes U-238, U-235, U-234 out of which U-238 is the most abundant and accounts for 99.3% of total natural uranium [5]. It is both a radiological and chemical toxic element. Primary organs effected by exposure to higher levels of U are bones and kidneys [6]. The chemical effect of U on the kidney are Nephritis and Kidney damage [7, 8] Further, the toxicity of U depends on many factors such as elimination route, exposure route, time of contact, solubility etc. [9]. The maximum permissible limit of U in drinking water given by various health organisations are different such as USEPA (United States Environmental Protection Agency) has defined the limit as 30 ppb [10], while WHO (World Health Organisation) has given limit as 30 ppb [11], whereas AERB (Atomic Energy Regulatory Board) in India has given the maximum permissible limit of 60 ppb that is twice of WHO and USEPA limit [12]. In the environment, uranium occurs in two different oxidation states namely hexavalent U(VI) and tetravalent U(IV). The hexavalent state is the most soluble state [13]. The oxidized uranium has more solubility and mobility [14]. The presence of U in groundwater has both natural and anthropogenic origin. Rocks such as organic rich shale, granite and rock-phosphate have uranium-bearing minerals for example pitchblende and uraninite [15]. The radionuclide is present in the crystal lattice of these minerals as a trace element [16]. The minerals in host rock upon contact with ground water, get dissolve in water and further on, interaction with sulphate, phosphate and carbonate ions in water form complexes and get transported [17, 18]. Anthropogenic causes involve the nuclear industry, emission, mill tailings release, coal combustion, utilisation of phosphate fertilisers and animal waste fertilisers [19, 20]. People are exposed to U through terrestrial exposure, inhalation and intake of food or water. The intake of water depends on many factors like sex, age, weight of the body, etc. Hence, for health risk assessment, monitoring of U concentration in drinking water is very much significant. Many risk analysis studies have been employed to trace radioactive elements and their products in nature [21, 22]. As these radionuclides cannot be geographically assessed by classical monitoring methods hence spatial modelling and distribution of radioactive compounds have been subject of many recent studies [14, 16, 22].
Several studies in India (Northern and Western parts) have reported the elevated level of uranium in drinking water and its potential health risk [20, 23,24,25]. In states like Punjab (500 ppb) and Rajasthan (133 ppb) very high concentration of uranium is found as a result of both anthropogenic and natural origins [24, 26]. Some studies have also been conducted on uranium concentration (ppb) in Himachal Pradesh (HP) like Kullu (0.07–2.35), Mandi (0.26–0.96), Bilaspur (1.79–4.65), Shimla (0.61–10.11), Kangra (0.98–6.14) [26,27,28,29].
The present study is an attempt to assess the concentration and spatial distribution of U in groundwater of Hamirpur district, Himachal Pradesh. The primary purpose of this research is to study the concentration and distribution of uranium in drinking water and to elucidate the possible health effects on the human population. Influence of various phsico-chemical parameters on uranium concentration and their correlation is also studied.
Study area and its geology
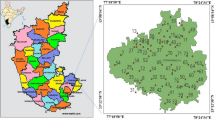
Nestled in the lap of Himalayas, Himachal Pradesh (HP) is situated in the north-western Himalayan region. It lies in hilly terrain between river Yamuna in the south-east and river Ravi in the north-west. The different agro-climatic zones found in HP are cold dry zone, Siwalik hill zone, mid and high hill zone. There are 12 districts in HP and the study was carried out in the district Hamirpur (Fig. 1). The district lies in Siwalik hill zone falling within 31°23′00″–31°53′00″N latitude and 76°20′00″–77°45′00″E longitude covering an area of 1118 sq. km. It is circumscribed on the south by Bilaspur, South-West by Una and northwest by Kangra district respectively. Generally, the elevation of the district varies from 400 to 1100 m above mean sea level. The average annual rainfall of the district is 340.72 mm. Main geological horizons include post-tertiary and tertiary formations. The entire district comprises of tertiary formations represented by Siwalik group of rocks. Aquifer system of the district comprises of sedimentary and porous formations. The later includes boulder beds, clays, sandstone and conglomerates while the former comprise of Fluviatile deposits that contain clay, sand, gravel, boulders and pebbles. Both in the conglomerate and sandstone formation, groundwater occurs in fractured or fault zone that further developed in the fashion of spring. The entire district has hand-pump having depth in the range of 40–80 m [30].
The whole district falls in mostly Siwalik range with some intercalations of Subhatu formations and alluvium. The Siwalik range is divided into three zones namely lower Siwalik, middle Siwalik and upper Siwalik as shown in Fig. 1. Lower Siwalik consist mainly of hard and compact, grey, greenish grey or purple sandstones that is fine to medium grained, with alternative clay clasts/bands. The clays are reddish brown to maroon in colour. At certain places cross bedding is also visible in lower Siwalik that are assigned an age of 18.3–10.8 Ma [31, 32]. The proportion of sandstone increases from lower to middle Siwalik succession [33]. The middle Siwalik consists of moderately compact and hard arkosic, medium to coarse grained grey sandstone interbedded with pebbles and thin clay beds. The individual sandstone bed generally varies from 2 to 10 m but total thickness can vary a lot at many places. In some places calcareous mudstone and silt stones are present that represent lacustrine conditions. The age of middle Siwalik varies from 10 to 5 Ma [34, 35]. Presence of massive conglomerates with thin bands of coarse grained sand bodies and reddish brown clays marks the occurrence of upper Siwalik. The frequency and thickness of conglomerate belts varies from place to place however it shows increasing up section. The age of upper Siwalik varies from 5 to 0.8 Ma [35]. It is interesting to note that the conglomerate of upper Siwalik contains pebbles and rock fragments of granite, Quartzite, Basic rocks, Limestone etc. in clay and rock flour matrix cemented by carbonate material [32]. Lower Siwalik are mostly present in the central portion of district (Hamirpur valley) and south eastern part of the district bordering Una and Bilaspur district. Thus, these areas are topographically at lower elevations as compared to middle Siwaliks which are present on the either side of Hamirpur valley (Fig. 1). Upper Siwalik zone is mostly located in the north eastern portion of district that has comparatively higher elevation and share the border with Mandi district. There are major two North east dipping thrusts passing through the district in addition to many lineaments/faults.
Experimental
Sample collection
The fieldwork has been carried out in the Hamirpur district in the month of May 2019. The district was divided into 6 × 6 km2 grid patterns to have systematic sampling of the entire area. From a single grid one sample was collected. Hence in all, 46 groundwater samples were collected from manually operated hand pumps, dug well, bore well, natural water and chashma/naalu, covering an area of approx. 1118 km2 as shown in Fig. 1. For sample collection and storage, the standard protocol given by BARC (Bhabha Atomic Research Center) is used that uses APHA guidelines. Each water sample is collected from one grid in polyethylene bottle that is soaked for overnight in 10% HNO3. All the samples were filtered using 0.45-micron filter paper. 1 litre of water is collected for the water quality analysis including parameters like F, Cl, NO3, SO4, PO4, and uranium. While 250 ml is collected for the analysis of hardness and alkalinity.
Physico-chemical analysis
The water samples were analysed for the physico-chemical parameters. These parameters play an essential role in evaluating the quality of water for potable and irrigation purposes. All the insitu parameters like pH, electrical conductivity, temperature, TDS, salinity, ORP (Oxidation Reduction Potential) and DO (Dissolved Oxygen) were measured properly on the field using multiparameter water kit (Orion STARA329, Thermo Scientific, Waltham, MA, USA). The kit was properly calibrated against the known standards. TH (Total hardness) and calcium hardness is calculated by standard titration method using Eriochrome Black and Paton & Reeder as indicators. To calculate magnesium hardness, the value of calcium hardness is subtracted from TH [36]. Similarly, alkalinity is calculated by standard titration method with standard H2SO4 solution and methyl orange as indicator. Both the hardness and alkalinity were measured within 24 h of sample collection. Ion-selective electrode is used for the measurement of fluoride and chloride using Orion STARA329, Thermo Scientific, Waltham, MA, USA instrument. Both the electrode were calibrated using known standards of fluorine (Fl) and chlorine (Cl). Further for the measurement of nitrate (NO3), phosphate (PO4) and sulphate (SO4) UV–Visible spectrophotometer (Hach dr 6000) is used. The outdoor environmental absorbed dose rate from terrestrial gamma radiation is measured using hand held radiation meter manufactured by Polimaster, PM 1405 survey meter. All the gamma radiation measurements were carried out at approximately 1 m distance from the ground at each of the locations. Five measurements were carried out at each sampling locations and mean of these value were taken to calculate effective dose for estimation of Annual Effective Dose (AED). All the measurements are done using the standard protocol shown by BARC during training and APHA, 2005 [37].
Determination of uranium
The concentration of uranium in the water sample is measured using Quantalase uranium analyser, which is based on the principle of Fluorescence being ignited by using LED Fluorimeter. The minimum detection limit of the instrument is 0.10 μg l−1 with an accuracy of ± 10% [4, 20, 25]. The LED generates intense and short lived pulses that cause the fluorescence of uranyl ion (UO22+) in the solution [38]. 5% Sodium pyrophosphate (Na4P2O7·10H2O) (SPP) solution is used as a fluorescence enhancing agent for the Quantalase instrument. The main function of SPP is to convert all the species of U to a single form, to increase the fluorescence of U, masking of quenchers like Fe and Mn and also to resist pH change by acting as a buffer. SPP is prepared using ultra-pure water by slowly adding diluted Phosphoric acid (H3PO4) (10% V/V) drop by drop to adjust its PH value to 7. The calibration of the instrument is done with a standard concentration of 1, 3, 5, 7 and 10 ppb prepared from the U standard of 100 ppm provided by Quantalase Enterprises Pvt. Ltd., Indore, India. Samples are analysed by standard addition method using a stock uranium solution of 500 ppb to nullify the matrix effect. Micro pipettes and analytical balance are used to avoid error in pipetting.
The values of U obtained for the groundwater samples was plotted in Geographic Information System environment using Arc GIS 10.4 software. The 46 water samples were interpolated using Inverse Distance Weight (IDW) method as it is best the method of interpolation when the data is collected in a grid pattern. The spatial distribution of uranium in the study area was plotted and analysed.
Chemo-radiological assessment
Uranium is a last naturally occurring heavy radioactive element that can be found in all the compartments of the environment. Both the carcinogenic as well as non-carcinogenic effects are associated via drinking of high level of uranium-contaminated water. Carcinogenic effects included the radiological risk which is associated with the radioactive property of uranium. While, the latter included chemical risk, as it is a heavy metal and affect kidney and lungs the most.
Radiological risk assessment
The carcinogenic effect as well as assessment of radiological risk is done in terms of ECR (Excess cancer risk). It is determined as the ingestion of radionuclide dose times the risk factor [39]. It is computed using Eq. (1):
where,
where r is uranium risk coefficient, IR is rate of water ingestion and EP is period of exposure. \(1\,\upmu{\text{g}}\,{\text{L}}^{ - 1} = 0.02528\,{\text{Bq}}\,{\text{L}}^{ - 1}\), conversion factor (r) of 1.19 × 10−9 Bq−1 is used for calculating U activity [40], IR is taken as 4.05 l day−1 [3] and EP is calculated by taking 65 years [11] and converting into days by including leap year days also (EP = 23740 days). However, the value of uranium in our study is well within the limit, and radiological risk is generally not estimated for such levels [41]. Hence ECR value is not estimated in this study. Further, it is suggested that, for lower values of U in groundwater, cancer risk should not be calculated and assumption for calculations may lead to statistical uncertainties.
Chemical toxicity evaluation
Uranium has several chemical toxic effects on the human body. Being a heavy metal, it gets accumulated in organs like kidney, liver and bones may cause potential health risk. Therefore, uranium chemical toxicity is calculated based on Lifetime Average Daily Dose (LADD) of U by consuming contaminated water. It is calculated using Eq. (3) [11];
UB = Concentration of U in environment (µg l−1), DIW = Daily water intake (4.05 l day−1), AD = Mean or average duration of exposure 65 years [11], LE = Life expectancy in days, 25201 days as per the recent world bank data of 2017 for India, considering leap years during whole expectancy of 69 years [42]. Since the LE changes with time, so recent LE available on World Bank data for India has been taken. ABW = Average weight of body, 53 kg (standard Indian person) [43], EF = Exposure frequency (350 days year−1) [33].
The effect of consumption of U contaminated water can further be calculated as Hazard Quotient (HQ). It is used for the estimation of harm that is produced through intake of uranium contaminated water [12]. HQ value less than 1, indicate no adverse effects on a person.
RD = Reference dose, 4.53 µg kg−1 day−1.
Annual effective dose (AED)
AED is calculated from outdoor absorbed gamma dose rate and an outdoor occupancy factor of 0.33. Its unit is mSv year−1 and is computed in Eq. (5) [44]
where Ad is outdoor absorbed dose measured in nSv h−1, CC is conversion coefficient (0.7 Sv Gy−1), OF is occupancy factor for outdoor exposure (0.33) which is calculated considering the exposure of 8 h per day for agricultural practices in hilly region and T is the time for 1 year in h (8760 h).
Water quality index (WQI)
The effect of all the parameters on the quality of water can be evaluated through a water quality index (WQI). In this study, WQI is calculated through weightage arithmetic index method [45]. It is calculated using the Eq. (6) given below; water having WQI value in the range 0–25 is considered as excellent water for drinking purpose, 26–50 as good quality water, 51–75, is poor quality water, > 100 is considered as unsuitable for drinking purposes [46]. pH, EC, TDS, TA, TA, Cl, F, NO3, Ca, Mg, SO4, are the parameters that were considered for calculating the WQI with WHO, 2011 standards (Table 1).
where Wn = unit weight of nth parameter, qn = sub-index of nth parameter or quality rating.
Further, qn and Wn is calculated using Eqs. 7 and 8;
where Cn = concentration of nth parameter, Sn = Permissible value, Cio = ideal value of nth parameter. Cio is considered as zero for all the parameters while for pH it is taken as 7 i.e. neutral water.
where K is proportionality constant and is computed as
Correlation analysis
Correlation analysis is a matrix based analysis which depicts the relationship among the chosen parameters. This helps in identifying those parameters which are either dependent (high correlation) or independent (low correlation) on the other parameters based on the data being given. The correlation between U and physico-chemical parameters has been studied in addition to analyse the interdependency of these parameter among themselves [4, 20, 25]. The concentration of U in ground water is dependent on the background water composition, rock types and the presence of uranium bearing minerals. Hence, to understand the possible cause and distribution of uranium, it is necessary to see its relationship with some major cations and anions such as carbonate, sulphate, chloride, phosphate and fluoride present in ground water. Also, oxidation reduction potential and oxic conditions control the concentration of U in ground water [5]. Thus in this study we used 14 parameters namely, pH, TDS, ORP, DO, Total Hardness (TH), Total Alkalinity (TA), Ca, Mg, F, Cl, PO4, NO3, SO4 and U, for estimation of correlation matrix.
Results and discussion
Physico-chemical
The statistical summary of physico-chemical parameters is given in (Table 2). The pH in the study area ranges from 6.26 to 8.52, with an average of 7.33. Electrical conductivity (EC) varies from 226 to 1308 μS/cm with an average of 685 μS/cm. EC of the water depends on total dissolved solids. Higher EC value is the result of dissolution and leaching of aquifer material [47]. Groundwater samples collected from hand-pump shows higher EC values as compared to water collected from springs. Generally, the groundwater sample has low dissolved oxygen (DO). The value of DO ranges from 1.1 to 7.73 mg/l with an average of 2.65 mg/l. TDS (Total dissolved solids) value ranges from 158 to 915 mg/l with an average of 479 mg/l. All the samples are within BIS permissible limit of 2000 mg/l, however 45% of the samples are above the BIS acceptable limit of 500 mg/l. ORP (oxidation–reduction potential) in the region lies between 112.6 and 376 mV, with an average of 249 mV. Total hardness recorded in the study area lies in the range of 75–500 mg/l with average of 289 mg/l. 84.7% of the samples are exceeding BIS acceptable limit of 200 mg/l. Hardness comes in the water samples as the result of dissolution of major cations such as Ca and Mg in groundwater. It is mainly attributed to the geologic formations and the host rock. The higher degree of hardness in the study area can be attributed to the higher soluble concentration of Ca2+, Mg2+, and HCO3− ions in aquifers system. Value of calcium and magnesium ranges from 16 to 124 mg/l and 8 to 63 mg/l with a mean of 63 and 31 mg/l respectively. However, at some location, the value of Mg hardness is more than the Ca hardness. About 41% of the samples are having magnesium ion concentration above BIS desirable limit of 30 mg/l. This may be due to precipitation of calcium after reaching super saturation and accounts for higher magnesium concentrations than calcium [47]. Also weathering of rocks like sandstone, dolomite, limestone, magnetite can be responsible for such high magnesium concentration. The study area lies in Siwalik formation which is dominated by sandstone, shale, limestone and dolomite type of rocks leading to higher concentration of Ca and Mg in water. Total alkalinity ranges from 112 to 562.5 mg/l with an average of 305.8 mg/l. 82% of the samples are exceeding BIS desirable limit of 200 mg/l. Value of fluoride and chloride ion ranges from 0 to 3 mg/l and 2 to 100 mg/l. Most of the samples are within the BIS permissible limit of F and Cl. Similarly, no significant concentration of phosphate is observed in the sample. The value of sulphate ranges from 8 to 99 mg/l with a mean of 65 mg/l. All the sample fall under BIS permissible limit of 400 mg/l of sulphate. Value of nitrate varies from 0 to 54.5 mg/l with an average of 11.56 mg/l. Out of 46 samples, 4 samples from, Garlic chowk (54.6 mg/l), Hamirpur (52.9 mg/l), Patla (51.4 mg/l), and Ladraur (50.1 mg/l) fall above WHO, 2011 permissible limit of 45 mg/l. Significant content of nitrate in water can cause several diseases like gastric cancer, blue baby syndrome and heart diseases [48]. Contamination of groundwater with nitrate is mainly due to agricultural activities, use of chemical and animal-derived fertilizers [48, 49]. Also, samples having higher nitrate content can be related to livestock and domestic waste near a water source. The high content of nitrate at above locations are most probably due to agricultural activities that are practiced in the valley fill zones near the river banks.
Spatial distribution of uranium
The concentration of uranium (U) in ground water was analysed and its spatial distribution was plotted using ArcGIS 10.4. The concentration varies between 0.15 and 18.92 μg l−1 in the study region with an average of 2.7 μg l−1. 26% of the samples fall above the average concentration of U in the study area with highest concentration found at 3 locations namely Hamirpur, Patla, and Chakkmoh. Details of concentration of uranium, uranium activity, ECR, HQ, and LADD of all the 46 groundwater samples are given in the (Table 3). The concentration of U in all the water samples is below the permissible limit prescribed by WHO [11] and USEPA [10]. However, the value of U obtained in the water sample of Hamirpur city found to be 2.23 times more than that reported by Singh et al. [23]. For example at Hamirpur From the spatial distribution map it can be interpreted that eastern part of district has lower concentration of uranium while the southern part has the higher concentration (Chakkmoh—12.45 ppb) as indicated with red color (Fig. 2). Minor concentrations of U are also observed in north-western part of the district. Further, the major concentration of U is observed along the NW–SE trendline with highest concentration in the central (Hamirpur—18.93 ppb) and south-eastern portion (Patla—15.53 ppb) of the district.
Generally, the presence of uranium in groundwater is the result of natural activity due to leaching from underlying bedrocks. Interestingly, the mineralization of uranium is reported in Hamirpur district [51, 52]. The entire region falls under Siwalik group of rocks having different lithology such as sandstone, conglomerates, shale etc. The Siwalik group of rocks have been explored for uranium mineralization by Atomic Minerals Directorate for Exploration and Research (AMDER) of Government of India since past 4 decades. These group of rocks shows different lithological characteristic that assist in uranium mineralization such as micaceous and immature feldspathic sandstones originated from fertile source, existence of porosity barriers and presence of pyrite and organic matter that act as reductant [32]. The rock fragments, pebbles, sidements of Siwalik formation are principally derived from Himalayan orogenic belt to the north and north east of the district. Three different uranium mineralization zone namely Hamirpur basin, Galot-SibaI-Loharkar tract and Romehra have been reported in the Hamirpur district [32]. The Hamirpur basin (Bhaleth-Astotha-Khya-Loharian-Karyali tract) lies in upper Siwalik, while Galot-SibaI-Loharkar tract and Romehra lies in middle and lower Siwalik respectively. The carbonaceous matter of sandtone in Hamirpur basin has significant concentration of U3O8 (4.861%) and Fe2O3 (81.42%) in it [23, 53]. The high amount of Fe2O3 is derived from pyrite in carbonaceous matter that further can act as a reductant and create suitable environment for uranium mineralization. We observed elevated concentration of U in groundwater of Hamirpur area that lies in the vicinity of mineralized zone of Astotha, Loharian, and Sibal. Usually the higher concentration of uranium and other radionuclides are associated with granites, volcanics phosphates and shale rocks [19, 48]. The conglomerates of Asthotha and Romehra has rock fragments of granite, quartzite, volcanics, limestone and argillites which are probable source for uranium mineralisation. Similarly, elevated concentration of U in groundwater of Patta and Chakkmoh is observed as these lie near Sibal and Rohmera uranium mineralized zone. Other minerals of uranium that are found in Asthotha and Khaya-Loharian tract are coffinite, uraninite, uranophane and schoepite. High concentration of uranium (1450 Bq kg−1) was found in soil samples of Hamirpur district as a result of uranium mineralization in the area [54].
Correlation analysis
Different parameters exhibit relations among each other which is studied in the form of correlation matrix. We used following mentioned 14 parameters namely, pH, TDS, ORP, DO, Total Hardness (TH), Total Alkalinity (TA), Ca, Mg, F, Cl, PO4, NO3, SO4 and U, for estimation of correlation matrix as shown in Table 4. Since EC, Salinity and TDS vary linearly and are interdependent, hence only TDS is taken for obtaining correlation matrix. However, correlation between 2 parameters is just not dependent on the concentration of the parameter but also on the confidence of the analysis as defined by p test. Hence, in addition to correlation, p value was also estimated and presented in Table 4. The cells lower than diagonal cells represent correlation values while those above the diagonal cells show the p value. The values less than 0.05 for p values indicates that the correlation value obtained is given with 95% confidence value. Hence those correlation values having p value less than 0.05 are considered significant in this study which are highlighted by green cells. Based on that it was observed that correlation values of more than 0.40 are quite significant. These are highlighted by blue cells in Table 4. The correlation values more than 0.60 is considered highly significant and are marked as bold. Highest correlation values are observed between total hardness with Ca and Mg ions as obvious, since they are inter- dependent.
Good correlation of TDS is observed between TH, TA, Mg, Cl, U and SO4. Further TDS shows higher correlation of 0.68 with Cl indicating leaching of anion from the rocks into the water table due to long travel time of water in the groundwater zone. Good correlation is observed between TA and Mg indicating the magnesium carbonate rock dissolution as the main reason for groundwater alkalinity [25].
Significant correlation of uranium is observed with TDS, TH, Cl, PO4 and NO3 (Table 4). Positive correlation is also observed with Ca, Mg and SO4. Highest correlation (0.62) of U is observed with TDS in this study area. Many ionic species are present in groundwater that have high TDS, which further can interact with uranium and finally cause enhancement in radioactivity [55]. The positive correlation of U was observed with Ca+2 and Mg+2 ions, indicating the speciation and mobilization of U in the form of Ca(UO2)(CO3)3 and Mg(UO2)(CO3)3 complexes [56]. Positive correlation of U with Mg and SO4 indicates the dissolution of Gypsum and Dolomitic limestone [4]. Also, the positive correlation of U with PO4 and SO4 may suggest the dissolution of uranium bearing minerals such as pitchblende, uraninite and their transport in the form of chemical complexes with phosphate and sulphate ions respectively [17, 18]. However, in this study area, the correlation of U with anions seems to be higher than cations. Good correlation of uranium with chlorine can be correlated with the occurrence of uranium-chloride in the form of dissolved salts [20, 57, 58]. This statistical finding indicates that the presence of uranium is partially correlated with the rock type and background water composition.
Higher concentration of nitrate is observed at those locations (Hamirpur, Ladraur, Chakkmoh etc.) where elevated concentration of U is also present. Thus, U shows higher correlation with NO3 as also reported by [57, 59]. It is noteworthy that these locations are situated in the vicinity of perennial water source. Due to presence of good water source, the population density increases. Hence denser population is present in the locations such as Hamirpur, Chakkmoh, Ladraur, Jahu, Patla where agriculture is their major occupation. To meet the demands of increasing population, agriculturist and farmers started utilization of more urea, animal manure and fertilizers. Excessive use of urea and animal based manures, increases the concentration of nitrate in water as seen in various location of the district. It is anticipated that with passage of time and utilization of excessive urea and fertilisers, these areas (Hamirpur, Ladraur, Chakkmoh etc.) will face more environmental problems due to nitrate contamination. Also, nitrate form complexes with uranium such as uranyl-nitrate, that is having strong solubility in groundwater [60]. Further, nitrate is considered to alter the solubility of uranium by dissolving its reduced U(IV) minerals oxidatively [61]. Hence, in future, the contamination of nitrate in the study area may increase the concentration of uranium in the ground water in addition to the geogenic origin.
Water quality index (WQI)
WQI is an important tool to identify the quality and sustainability of water for drinking purpose [62]. For monitoring the quality of water, these water quality indices are considered as communication and aggregation tools [63]. The calculated WQI ranged from 1.28 to 168.83 with an average of 12.00 for 46 groundwater sample (Table 3). Individual WQI value for every sample is given in Table 1. Nearly all the water samples fall under the excellent quality of water except only one sample that falls in unsuitable category for drinking purposes. The high concentration of fluorine is observed in that sample leading to its higher WQI value (168.83). Thus the quality of groundwater in the study area seems to be suitable for drinking purposes, however at certain specific pockets, concentration of uranium and anthropogenic activities needs to be checked so that concentration of nitrate and uranium remains within the acceptable limit.
Assesment of chemical toxicity and outdoor dose rate
The Lifetime Average Daily Dose (LADD) value ranges from 0.01 to 1.31 μg kg−1 day−1 with an average of 0.19 μg kg−1 day−1. LADD shows a wide variety of value, as the concentration of uranium is not uniformly distributed. The value of LADD lies within the permissible limit of 4.53 µg kg−1 day−1 [12]. Further to determine the chemical toxicity, HQ is computed. The high value of HQ greater than 1 indicates the unsuitability of water for drinking purpose. In the present study, HQ ranges from 0.002 to 0.288 with the mean value of 0.04, that indicates the suitability of groundwater for drinking purposes. Since HQ is dependent in LADD, hence in both the computations, 26% of the samples fall above the average value of LADD and HQ.
The absorbed dose rate from outdoor terrestrial gamma radiation ranges from 60 to 135 nSv h−1 with an average of 102 nSv h−1. 43% of the samples fall above the average absorbed dose in the study area with highest value found at locations Daragon (135 nSv h−1), Barara (130 nSv h−1), Patla (125 nSv h−1), Bhota (125 nSv h−1), and Chamoh (120 nSv h−1) respectively. The variation of outdoor gamma radiation for various locations follows a similar trend as that of uranium concentration in water samples as shown in Fig. 3. The locations where lower uranium concentration is observed in water, also shows lower values of gamma radiation and similarly for elevated values. However, a contrasting pattern is shown for sample collected at Hamirpur city. At this location the concentration of U in groundwater is higher than other locations and so higher gamma radiation is expected, but comparatively lower gamma radiation is observed in this location. This could be attributed to the fact that in Hamirpur city, underground rocks and soil are not exposed at the location where sampling was carried out, hence the gamma radiation is less.
The value of AED calculated from absorbed dose rate ranges from 0.12 to 0.27 mSv year−1 with an average of 0.21 mSv year−1, which is higher than the world average of 0.07 mSv year−1 [64] but are below the background radiation level of (2.4 mSv year−1). Comparatively higher AED values in the study area is observed at Daragon and Barara. Slightly higher background radiation level in the study area can be the result of radionuclide present in the parent rocks. Similarly, the value of high terrestrial gamma radiation and AED at location Daragon and Barara is may be due to radionuclides present in soil and rocks as less concentration of uranium present in ground water at these locations. However, in depth analysis of radioactivity in soil and host rocks needs to be carried out to ascertain some concrete results in the study area.
Conclusion
The present investigation determined the spatial distribution, radiological assessment, concentration of uranium and other water quality parameters in groundwater of Hamirpur district, Himachal Pradesh. Most of the water quality parameters are within permissible limit except nitrate, magnesium and alkanility. The value of HQ and WQI indicates that the suitability of water for drinking purpose. The region has minute natural terrestrial radioactivity and are well below than the background radiation. However, the average value of AED is 0.21 mSv year−1, which is thrice the world average of 0.07 mSv year−1. The measured concentration of uranium ranges from 0.15 to 18.92 μg l−1 in the study region with an average of 2.7 μg l −1 which is below the WHO permissible limit. Spatial distribution of uranium shows that the major concentration of uranium is present along the NW–SE trendline. At few locations which are densely populated (Hamirpur, Chakkmoh and Patla) elevated concentration of uranium is observed as also found by other investigators, hence it is necessary to periodically monitor the ground water of this region and to investigate the main cause and origin of this contamination. This elevated concentration of uranium is observed mostly in Siwalik range where mineralisation is prevalent. The granitic and metamorphic rock fragments present in the conglomerates of the area are expected to be the geogenic source. Higher positive correlation of uranium is also observed with nitrate, and chlorine indicating the influence of agriculture activities in addition to leaching of uranium from host rocks. The higher nitrate values is observed in densely populated areas as a result of agricultural activities. Thus there is a need to educate the farmers about these current scenarios and inform them about the feasible utilization of the fertilizers and manures. Proper knowledge needs to be provided to them about utilising better and alternative water management practices.
References
Yadav AK, Sahoo SK, Mahapatra S, Kumar AV, Pandey G, Lenka P, Tripathi R (2014) Concentrations of Uranium in drinking water and cumulative, age-dependent radiation doses in four districts of Uttar Pradesh, India. Toxicol Environ Chem 96:192–200
Duggal V, Sharma S (2017) Chemotoxicity and radiotoxicity risk assessment from exposure to Uranium in groundwater from western Haryana, India. Int J Pure Appl Phys 13:07–112
Saini K, Singh P, Bajwa BS (2016) Comparative statistical analysis of carcinogenic and non-carcinogenic effects of Uranium in groundwater samples from different regions of Punjab, India. Appl Radiat Isot 118:196–202
Kumar D, Singh A, Jha RK (2018) Spatial distribution of Uranium and basic water quality parameter in the capital of Bihar and consequent ingestion dose. Environ Sci Pollut Res 25(18):17901–17914
Smedley P, Smith B, Abesser C, Lapworth D (2006) Uranium occurrence and behaviour in British groundwater. British Geological Survey Commissioned Report, CR/06/050 N. 60 pp
Craft ES, Abu-Qare AW, Flaherty MM, Garofolo MC, Rincavage HL, Abou-Donia MB (2004) Depleted and natural Uranium: chemistry and toxicological effects. J Toxicol Environ Health 7(4):297–317
Llobet JM, Sirvent JJ, Ortega A, Domingo JL (1991) Influence of chronic exposure to Uranium on male reproduction in mice. Toxicol Sci 16(4):821–829
Vicente L, Quiros Y, Barriocanal F, Novoa J, Hernandez F, Morales A (2010) Nephrotoxicity of Uranium: pathophysiological, diagnostic and therapeutic perspectives. Toxicol Sci 118(2):324–347
ATSDR (1999) Toxilogical profile for Uranium. Agency for Toxic Substances and Disease Registry, Atlanta
USEPA (2011) United States environmental protection agency, edition of the drinking water standards and health advisories. Office of Water, Washington DC
WHO (2011) Guidelines for drinking water quality, vol 1, 4th edn. World Health Organisation, Geneva
AERB (Atomic Energy Regulatory Board) (2004) Drinking water specifications in India. Department of Atomic Energy, Govt. of India
Cothern CR, Lappenbusch WL (1983) Occurrence of Uranium in drinking water in the US. Health Phys 45(1):89–99
Külahcı F, Bilici A (2019) Advances on identification and animated simulations of radioactivity risk levels after Fukushima Nuclear Power Plant accident (with a data bank): a critical review. J Radioanal Nucl Chem 321(1):1–30
Keith LS, Faroon OM, Fowler BA (2007) Uranium. In: Berlin M, Zalups RK, Fowler BA (eds) Handbook on the toxicology of metals. Academic Press, Burlington, pp 880–903
Erenturk S, Yusan S, Turkozu DA, Camtakan Z, Olgen MK, Aslani MA, Aytas S, Isik MA (2014) Spatial distribution and risk assessment of radioactivity and heavy metal levels of sediment, surface water and fish samples from Lake Van, Turkey. J Radioanal Nucl Chem 300(3):919–931
Wanty RB, Schoen R (1992) A review of the chemical processes affecting the mobility of radionuclides in natural waters, with applications. In: Gundersen LCS, Wanty RB (eds) Field studies of radon in rocks, soils, and water. CRC Press, Boca Raton, p 183
Murphy WM, Shock E (1999) Environmental aqueous geochemistry of actinides. Rev Mineral Geochem 38:221–253
Spalding RF, Sackett WM (1972) Uranium in runoff from the Gulf of Mexico distributive province: anomalous concentrations. Science 175(4022):629–631
Sharma T, Sharma A, Kaur I, Mahajan RK, Litoria PK, Sahoo SK, Bajwa BS (2019) Uranium distribution in groundwater and assessment of age dependent radiation dose in Amritsar, Gurdaspur and Pathankot districts of Punjab, India. Chemosphere 219:607–616
Külahcı F, Çiçek Ş (2019) On the determination of transportation, range and distribution characteristics of Uranium-238, Thorium-232 and Potassium-40: a critical review. Environ Earth Sci 78(24):721
Kumar A, Karpe R, Rout S, Joshi V, Singhal RK, Ravi PM (2013) Spatial distribution and accumulation of 226 Ra, 228 Ra, 40 K and 137 Cs in bottom sediments of Mumbai Harbour Bay. J Radioanal Nucl Chem 295(2):835–839
Singh P, Sahoo BK, Bajwa BS (2016) A study on Uranium and radon levels in drinking water sources of a mineralized zone of Himachal Pradesh, India. J Radioanal Nucl Chem 309(2):541–549
Bajwa BS, Kumar S, Singh S, Sahoo S, Tripathi R (2017) Uranium and other heavy toxic elements distribution in the drinking water samples of SW-Punjab, India. J Radiat Res Appl Sci 10:13–19
Diwan V, Sar SK, Biswas S, Dewangan R, Baghel T (2019) Uranium in ground water of Rajnandgaon District of Central India. J Radioanal Nucl Chem 321(1):293–302
Rani A, Singh S, Duggal V, Balaram V (2013) Uranium estimation in drinking water samples from some areas of Punjab and Himachal Pradesh. India using ICP-MS. Radiat Prot Dosim 157(1):146–151
Singh B, Singh G, Sandhu AS, Singh S (1999) Uranium estimation in water samples collected from some areas of Himachal Pradesh, India. Radiat Meas 31:683–685
Bajwa BS, Mahajan S, Singh H, Singh J, Singh S, Walia V, Virk HS (2005) A study of ground water radon concentrations in Punjab and Himachal Pradesh states, India. Indoor Built Environ 14:481–486
Singh J, Singh H, Singh S, Bajwa BS (2008) Estimation of Uranium and radon concentration in some drinking water samples. Radiat Meas 43:523–526
CGWB (Central Ground Water Board) (2013) Ground water information booklet, Hamirpur district, Himachal Pradesh. http://cgwb.gov.in/District_Profile/HP/Hamirpur.pdf. Accessed 15 July 2019
Johnson NM, Stix J, Tauxe L, Cerveny PF, Tahirkheli RA (1985) Paleomagnetic chronology, fluvial processes, and tectonic implications of the Siwalik deposits near Chinji village, Pakistan. J Geol 93(1):27–40
Kaul R (1993) Uranium mineralization in the Siwaliks of North Western Himalayan, India. J Geol Soc India 41:243–258
Kumaravel V, Sangode SJ, Kumar R, Siddaiah NS (2005) Magnetic polarity stratigraphy of Plio–Pleistocene Pinjor Formation (type locality), Siwalik Group, NW Himalaya, India. Curr Sci 88:1453–1461
Barndt J, Johnson NM, Johnson GD, Opdyke ND, Lindsay EH, Pilbeam D, Tahirkheli RAH (1978) The magnetic polarity stratigraphy and age of the Siwalik Group near Dhok Pathan village, Potwar Plateau, Pakistan. Earth Planet Sci Lett 41:355–364
Sangode SJ, Kumar R (2003) Magneto stratigraphic correlation of the Late Cenozoic fluvial sequences from NW Himalaya, India. Curr Sci 84:1014–1024
Taras M, Greenberg A, Hoak R, Rand M (1971) Standard methods for the examination of water and wastewater, 13th edn. American Public Health Association, Washington
APHA (American Public Health Association), (2005) Standard methods for the examination of water and wastewater. APHA, Washington
Sahu SK, Maity S, Bhangare RC, Pandit GG, Sharma DN (2014) Determination of Uranium in ground water using different analytical techniques (No. BARC–2014/E/011). Bhabha Atomic Research Centre, Mumbai
Patra A, Mohapatra S, Sahoo S, Lenka P, Dubey J, Tripathi R, Puranik V (2013) Age-dependent dose and health risk due to intake of Uranium in drinking water from Jaduguda, India. Radiat Prot Dosim 155:210–216
USEPA (United States Environmental Protection Agency) (1999) Cancer risk coefficients for environmental exposure to radionuclides. Federal Guidance Report No. 13, EPA 402-R-99-001
Burk B (2019) Radiation risk in perspective. Position statement of the health physics society. https://hps.org/documents/radiationrisk.pdf, Accessed 5 Nov 2019
World Bank (2017) The world development indicators, World BankPublications, Washington. https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=IN. Accessed 9 Dec 2019
Sharma S, Kumar A, Mehra R, Mishra R (2017) Ingestion doses and hazard quotients due to intake of Uranium in drinking water from Udhampur District of Jammu and Kashmir State, India. Radioprotection 52:109–118
Ajayi OS (2009) Measurement of activity concentrations of 40 K, 226 Ra and 232 Th for assessment of radiation hazards from soils of the southwestern region of Nigeria. Radiat Environ Biophys 48(3):323–332
Brown RM, McClelland NI, Deininger RA, Tozer RG (1970) Water quality index-do we dare? Water Sewage Works 117(10):339–343
Thakur N, Rishi M, Sharma DA, Keesari T (2018) Quality of water resources in Kullu Valley in Himachal Himalayas, India: perspective and prognosis. Appl Water Sci 8(1):20
Hem JD (1991) Study and interpretation of the chemical characteristics of natural water: USGS professional paper book 2254. Scientific Publishers, Jodhpur
CPCB (Central Pollution Control Board) (2008) Guideline for water quality management. CPCB, Parivesh Bhawan, East Arjun Nagar, Delhi
Wang Z, Lee SW, Kapoor P, Tebo BM, Giammar DE (2013) Uraninite oxidation and dissolution induced by manganese oxide: a redox reaction between two insoluble minerals. Geochim Cosmochim Acta 100:24–40
BIS (Bureau of Indian Standards) (2012) Drinking water specification. Bureau of Indian standards, New Delhi (IS 10500)
Kumar J, Malhotra R, Singh J, Singh S (1994) Radon measurements in dwellings in radioactive areas in Himachal Pradesh, India, using LR-115 plastic track detectors. Nucl Geophys 8(6):573–576
Sharma M, Sharma YC, Basu B, Gupta RK, Singh J (2000) Uranium mineralization in the sandstones of Dharamsala, Tikli area, Mandi-district, Himachal Pradesh, India. Curr Sci 78(7):897–899
Bonotto DM (2014) 222Rn, 220Rn and other dissolved gases in mineral waters of southeast Brazil. J Environ Radioact 132:21–30
Virk HS (1997) Uranium and radon surveys in western Himalaya. Curr Sci 73(6):536–538
Ortega X, Valles I, Serrano I (1996) Natural radioactivity in drinking water in Catalonia (Spain). Environ Int 22:347–354
Fox PM, Davis JA, Zachara JM (2006) The effect of calcium on aqueous Uranium(VI) speciation and adsorption to ferrihydrite and quartz. Geochem Cosmochim Acta 70:1379–1387
Singh S, Rani A, Mahajan RK, Walia TPS (2003) Analysis of Uranium and its correlation with some physico-chemical properties of drinking water samples from Amritsar, Punjab. J Environ Monit 5:917–921
Selvi B, Vijayakumar B, Rana B, Ravi P (2016) Distribution of natural Uranium in groundwater around Kudankulam. Radiat Prot Environ 39:25
Buro KR, Nolan BT, Rupert MG, Dubrovsky NM (2010) Nitrate in groundwater of the United States, 1991 − 2003. Environ Sci Technol 44(13):4988–4997
Schnug E, Lottermoser BG (2013) Fertilizer-derived Uranium and its threat to human health. Environ Sci Technol 47(6):2433–2434
Nolan J, Weber KA (2015) Natural Uranium contamination in major US aquifers linked to nitrate. Environ Sci Technol Lett 2(8):215–220
Magesh NS, Krishnakumar S, Chandrasekar N, Soundranayagam JP (2013) Groundwater quality assessment using WQI and GIS techniques, Dindigul district, Tamil Nadu, India. Arab J Geosci 6(11):4179–4189
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K, Rajiv Ganthi R, Chidambaram S, Anandhan P, Manivannan R, Vasudevan S (2010) Application of water quality for groundwater quality assessment: Thirumanimuttar sub basin, Tamil Nadu, India. Environ Monit Assess 171(1–4):595–609
UNSCEAR (United Nations Scientific Committee on the Effect of Atomic Radiation) (2000) Sources and effects of ionizing radiation. United Nations, New York
Acknowledgements
Authors are grateful to BRNS (P/no-IITM/DAE-BRNS/DPS/188) for financing this research work. We are also thankful to IIT Mandi for providing lab facilities for conducting various experiments for the analysis of the samples. We thank the anonymous reviewers for their constructive comments which has shaped the manuscript in the better form.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhardwaj, S., Shukla, D.P. & Halder, A. Spatial distribution of uranium and chemo-radiological assessment in Hamirpur district, Himachal Pradesh, India. J Radioanal Nucl Chem 324, 467–480 (2020). https://doi.org/10.1007/s10967-020-07088-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07088-7