Abstract
We have developed a new chromatographic method to efficiently separate and isolate neptunium (Np) and protactinium (Pa), based on the selective extraction of protactinium by primary alcohols. The effectiveness of the new technology is demonstrated by efficient separation of 233Pa from parent radionuclide 237Np, using a hydrochloric acid mobile-phase medium. Our new approach reproducibly isolated 233Pa tracer with a yield of 99 ± 1 % (n = 3; radiochemical purity 100 %) and enabled chemical recovery of 237Np parent material of 92 ± 3 % (radiochemical >99 %) for future 233Pa tracer preparations. Compared to previous methods, the new approach reduces radioactive inorganic and organic waste; simplifies the separation process by eliminating cumbersome liquid–liquid extractions; and allows isolation of radiochemically-pure fractions in less than 1 h.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Investigations to understand the chemical properties of the rare actinide (An) elements protactinium (Pa; Z = 91; [Rn]5f 2 6d 1 7s 2) and neptunium (Np; Z = 93; [Rn]5f 4 6d 17 s 2) over the past few decades have led to a greater understanding of the physico-chemical properties of the 5f-group An. The chemistry and applications of these elements are fascinating and diverse [1, 2]. For example, Pa isotopic signatures and radioactive steady-state relationships can provide important information for geologists [3–6]; nuclear forensic scientists [7–9]; and nuclear engineers [10]. However, deficiencies in our understanding of Pa chemistry often complicate radioanalytical methods employed for these investigations [6, 11, 12].
While many of these complications arise from limited accessibility to appreciable quantities of Pa for spectroscopic studies, expanding investigations into Pa separations and purifications have led to exciting new applications. For example, efficient analysis of Pa-bearing nuclear materials for applications in nuclear forensics and Th-based nuclear fuel development are leading to an increasing need for technologies that enable isolation of radiochemically-pure Pa from complex and (in some cases) high-radiation-field media [12, 13]. Specifically, beta-emitting Pa radionuclide, 233Pa (t 1/2 = 26.967 days), may be an undesirable contaminant in uranium-233 (233U) nuclear fuels because of its large neutron cross section [14]. Within this same context, nuclear forensic and geochronological analysis of long-lived 231Pa (t 1/2 = 3.276 × 105 years) by isotope dilution techniques generally requires the use of 233Pa as a yield monitor for accurate determinations of 231Pa by alpha spectrometry and mass spectrometry [6, 7, 15]. Interestingly, research investigators published the most comprehensive reports on Pa chemistry during the 1950s and 1960s, and anticipated that “much of mystery and witchcraft [of protactinium chemistry]” would be eliminated with the advent of appropriate tracer preparation techniques [16, 17]. Ironically, to this day, much of the mystery of Pa remains unresolved, due in part to the need for more effective strategies for the preparation of isotopic tracers [11]. Thus, there is a critical need to develop robust technologies for separating and isolating Pa from materials.
Like Pa, isotopes of Np are of interest for applications in environmental science; nuclear engineering; and as part of isotopic signatures in nuclear materials for forensic applications [18]. While identification of isotopes of Pa occurred in the early part of the twentieth century, it was not until the 1940s that investigators isolated sufficient quantities of Np radionuclides to confirm its predicted-basic electronic and chemical properties [2]. Of the isotopes of Np, alpha-emitting radionuclide 237Np (t 1/2 = 2.14 × 106 years) is of significant interest because it has been identified as an environmental concern and as a radionuclide that has the potential to assist in nuclear forensic analysis of materials [9, 19]. In environmental science, 237Np(V) is highly mobile in subsurface systems and is considered a problematic radionuclide for the long-term storage of high-level wastes produced from nuclear fuel cycle and weapons development. Worries regarding effectiveness of engineered barriers and accidental release have led to research into the mechanism of Np adsorption and transport in biogeochemical systems [18, 20]. While the relatively long half-life of 237Np is one rationale for concern in terms of its fate, transport, and potential for bioaccumulation, the half-life and easily-detected alpha emissions of 237Np also enable its use for studies of fundamental Np properties [2].
On the other hand, investigations of the chemical properties of Np using 237Np are often complicated by radioactive ingrowth of its immediate radionuclide decay product, 233Pa, and current separations technologies are cumbersome for routine analyses [21]. Due to a relatively short half-life, the observed radioactivity of 233Pa increases rapidly toward secular equilibrium (steady state) with 237Np, which is undesirable for many applications. Conversely, the rapid ingrowth of 233Pa facilitates the use of calibrated 237Np standard solutions for the preparation of 233Pa tracer for isotope dilution analysis of 231Pa in materials, as described above. Consequently, researchers must routinely remove 233Pa from 237Np sources [15, 16, 22]. Unfortunately, current methods to isolate 233Pa from 237Np involve anion-exchange (e.g., AG-MP1 [15], Dowex-1 [17], AG1-X8 [6] ) and liquid–liquid extraction (e.g., diisobutyl ketone [15], diisobutyl carbinol [17] ) techniques that generate substantial liquid (organic and inorganic) wastes—and often employ potentially hazardous hydrofluoric acid (HF) [6, 15, 22]. Although HF is an effective complexing agent for Pa, which allows back-extraction into the aqueous phase [16], excess F− can prevent Pa extraction in subsequent steps [6]. Furthermore, HF poses a serious health hazard and should be avoided when possible [23].
Within this context, we have developed a new technology and approach for the efficient separation and purification of Pa and Np that will be beneficial for the preparation of 233Pa tracer from 237Np for isotope dilution radioanalytical analysis [15, 16, 24], and for the removal of 233Pa from 237Np for research investigating fundamental and environmental Np chemistry [18]. In this article, we describe this new and highly efficient separation of Np and Pa, which is based on selective extraction of Pa by 1-octanol in hydrochloric acid (HCl) media. Our new method reproducibly isolated 233Pa tracer with a yield of 99 ± 1 % (n = 3; radiochemical purity 100 %) and enabled chemical recovery of 237Np parent material of 92 ± 3 % (purity >99 %) for future 233Pa tracer preparations. Compared to previous methods, our new approach significantly reduces radioactive inorganic and organic waste; simplifies the separation process by eliminating liquid–liquid extraction required by previous methods; and reduces the time required—yields radiochemically-pure fractions of Pa and Np in less than 1 h. We anticipate this method can be further adapted for numerous applications and desired experimental conditions.
Experiemental
General
Chemical regents for radiochemical separation and source preparation (HCl), ammonium hydroxide (NH4OH), 35 % hydrogen peroxide (H2O2), and bromocresol purple were ACS regent grade (Fisher Scientific) or higher. A calibrated source (1000 μg mL−1) of cerium (Ce) (chloride form) was used for micro-precipitation of radionuclide sources (High Purity Standards, Charleston, SC). Radioactivity standards were prepared in Ultra-pure HCl (Fisher Scientific) and diluted with ultra-pure distilled deionized water (Baseline®, Seastar Chemicals, British Columbia, Canada), both certified to parts-per-trillion (ppt) trace metals content. Half-lives and alpha-particle/gamma-ray energies are values originating from the Evaluated Nuclear Structure Data File (ENSDF) that were obtained through the United States National Nuclear Data Center (NNDC, Brookhaven National Laboratory, US Department of Energy) [25]. All uncertainties are “standard uncertainties” corresponding to a coverage factor k = 1 [26], unless explicitly stated otherwise.
Safety considerations
Use of radioactive materials is potentially hazardous and appropriate ALARA principles should be considered prior to conducting experiments using radioactive materials. Both 233Pa (beta-particle and gamma-ray emissions) and 237Np (alpha-particle and gamma-ray emissions) are radioactive isotopes and should be used only in facilities designed to handle radioactivity.
237Np and 233Pa sources
The radiation solution standards of 237Np (Reference Numbers 92566, 96584, 93498) used for this study were purchased from the Eckert and Ziegler Radioisotopes (Atlanta, GA, USA). Standards were used after at least 7 months of the reference date to allow for ingrowth of radioactive decay product 233Pa. The material has been certified to include a minor alpha-emitting impurity (238Pu) [9]. To prepare working solutions, the glass ampoule (5 mL in 0.5 M HNO3) was scored at the neck and broken—and the contents were transferred to a pre-acid-leached beaker (25 mL). The contents were taken dry slowly, and re-dissolved in ultra-pure 6 M HCl (Seastar Chemicals, British Columbia, Canada). This process was repeated four times to an apparent complete matrix conversion to the chloride form; and finally redissolved to provide a working stock solution in 25 mL ultra-pure 6 M HCl (~145 Bq mL−1 237Np and 233Pa). Final solutions were transferred and continuously stored in a metals-grade ultra-pure Seastar (Seastar Chemicals, Canada) Teflon bottle at 5 °C to prevent evaporation.
Resin preparation
The primary-alcohol extractant 1-octanol was chosen (based upon previous methods) to prepare the 233Pa radiometric tracer [16, 24]. The resin form of 1-octanol used for the experiments was prepared using a procedure developed by Eichrom Technologies, Inc. (Lisle, IL USA) [27]. Briefly, 1-octanol (10 g) was dissolved into methanol (100 mL) then mixed with the resin beads (15 g; Amberchrom CG71, 25–50 μm, Rohm and Haas, Philadelphia, PA USA). The mixture was stirred (1 h) in a rotary evaporator, and then the methanol was removed under vacuum at room temperature. The resulting material was 40 % (w:w) 1-octanol (verified by thermogravimetric mass analysis). This material is not commercially available, but can be made available on request at no cost by the authors. The material was prepared approximately 1 year prior to these investigations. Qualitatively consistent results have been produced during this time, which reflect the stability of the material stored at room temperature under typical laboratory conditions.
Columns were prepared by a previously described routine procedure [28]. Briefly, a slurry (0.66 g per 5 mL) was homogenized and transferred to an empty 2 mL column (AC-141-AL, Eichrom) allowing the water to drain by gravity flow. The column was secured with pre-manufactured frits (provided with empty columns) and a 25 mL reservoir (AC-120, Eichrom). The column was then preconditioned (10 mL of 9 M HCl) prior to loading of the 237Np/233Pa solution (Fig. 1).
Protocol used to separate Pa and Np using a resin form of 1-octanol. The column is preconditioned in 9 M HCl (5 mL) and the sample is loaded on the column in 9 M HCl (5 mL). Neptunium is collected together with the eluent of the load solution and a rinse of 9 M HCl (30 mL). Protactinium is eluted from the column with 1 M HCl (30 mL). Radiochemically pure fraction of 233Pa and 237Np were analyzed separately by gamma and alpha spectroscopy
Separation protocol
To separate 233Pa and 237Np, aliquots of the calibrated standard solution (100 μL, n = 3) that contained 14.5 Bq of both 237Np and 233Pa (in secular equilibrium) were transferred to a liquid scintillation (LS) vial (25 mL), diluted to 1 mL with 9 M HCl, and added to the column. The eluent of the load solution was collected for 237Np analysis. Next, the LS vial was rinsed (4 mL, 9 M HCl) and added to the column. The column was then rinsed directly with 30 mL 9 M HCl to wash any remaining 237Np that was retained on the column. All solvent fronts of 9 M HCl were collected together in an LS vial (50 mL) to be analyzed for 237Np yield and purity. Following the removal of 237Np, 233Pa was eluted by passing 30 mL of 1 M HCl through the column. The eluent was collected in another LS vial (50 mL) to be analyzed for 233Pa yield and purity. Each liquid LS vial was precisely “time-stamped” to the time that the final drop was collected into the LS vial. This time-stamp was used as the reference to correct for the radioactive decay of 233Pa activity. An internal standard was prepared containing the same amount of radioactivity from the stock solution (100 μL, 14.5 Bq; 237Np and 233Pa) of the solution standard in an identical geometry with HCl (30 mL) in a LS vial (50 mL).
Gamma spectrometry
Immediately following the separation process, the sources were counted by routine procedures using high-purity germanium (HPGe) gamma-ray spectrometry and analyzed using the gamma spectrometry software GammaVision (Ortec, Oak Ridge, TN) [29]. The internal standard was counted for 4 h and used to calibrate the energy and efficiency of the 237Np and 233Pa peaks (using 86.47 and 311.90 keV respectively) (Table 1). A a matched count-time blank background spectra was collected using an LS vial with 30 mL HCl to account for background spectral corrections. The detection limit was calculated to be 2.4 mBq (0.01 % of the total activity) [26]. Each sample was counted for 4 h and “time-stamped” at the acquisition time, which was used to determine the total elapsed time between the end of the separation and the starting of the HPGe analysis. All of the sources were counted within 48 h of separation, to ensure that (233Pa)time/(233Pa)initial > 95 %, allowing for negligible effect of the decay constant to the overall uncertainty of the Pa measurement.
Source preparation and alpha spectrometry
To assess the yield and purity of Np and Pa fractions, 233Pa and 237Np alpha-counting sources were prepared by cerium hydroxide micro-precipitation and were analyzed by alpha spectrometry by previously described routine procedures [28]. Briefly, the contents were transferred to a beaker together with Ce carrier (50 μg), H2O2 (500 μL), and bromocresol purple pH indicator. Note: H2O2 and 9 M HCl will fade the color, so addition of more bromocresol is acceptable to ensure the final pH range is determined correctly. The pH was then increased with the addition of concentrated NH4OH to pH ~ 8, where the presence of the indicator resulted in a purple solution. The mixtures were set aside for 10–20 min to allow for the microprecipitate to form. In the meantime, a filtration apparatus was assembled on a vacuum box and lined with 0.1 μm Resolve Filters™ (RF-100-25PP01, Eichrom) that were pre-wetted with 80 % ethanol. The samples were then filtered via vacuum filtration and allowed to dry for 30 min with the vacuum pump on. Once dry, the filters were mounted on stainless steel planchettes (31.75 mm outer diameter, AF Murphy, Quincy, MA, USA). Finally, to prevent contamination of the alpha detectors resulting from daughter recoil, thin films consisting of iso-amyl acetate and collodion were placed directly on the source as we described previously [30].
All alpha spectra were collected with 450 mm2 passivated ion-implanted silicon detectors in vacuum controlled-alpha spectrometers (Alpha Analyst, Canberra, Meridan, CT) with a fixed source to detector distance of about 10 mm. Efficiencies are determined for each detector every 6 months using a standard of identical geometry (Eckert and Ziegler; SRS: 91005; 238U, 234U, 239Pu, and 241Am) and range from ~17 to 20 % depending on which detector is used. For each measurement the efficiency of the detector used for an individual measurement is used to determine the radioactivity. Alpha sources of the 237Np sources were counted for about 24 h (>100,000 counts) contributing <0.3 % uncertainty from counting statistics. The 233Pa sources were counted for 100 h to determine presence of trace 237Np. In each 233Pa source, presence of trace 237Np achieved >500 counts attributing to <4.5 % uncertainty from counting statistics. A matched count-time background was obtained and subtracted from the 237Np region of interest (ROI) (Table 1).
Liquid scintillation counting
To determine the elution peak maximum values for 237Np in 9 M HCl and 233Pa in 9 M HCl and 1 M HCl, samples were counted by LS counting. For LS counting experiments, a Packard 1600 CA Tri-Carb (Perkin Elmer, Waltham, MA) was used with EcoLite LS cocktail in 30 mL glass LS vials with a water fraction of 10 %. The samples were counted by routine lab procedure [28].
Results and discussion
General
This paper presents a novel approach to the chemical separation and isolation of radiochemically-pure fractions of Pa and Np that is based on selective extraction of Pa by aliphatic primary alcohols—using a new chromatographic-resin material and 1-octanol as the extractant-functional moiety. We prepared batches of the 1-octanol extraction chromatographic resin via standard evaporative techniques and evaluated the new approach using a typical gravity-flow column-chromatography arrangement. Excellent separation and radiochemical isolation of 233Pa and recovery of parent 237Np were observed at radioactivity concentrations of 233Pa tracer (14.5 Bq/sample) typical to environmental radiochemistry laboratories employing 233Pa tracer for isotope dilution alpha spectrometry analysis of 231Pa in environmental samples. We anticipate that the method could be modified to accommodate elevated levels of 237Np for other applications.
The procedure comprised four basic chromatographic steps (Figs. 1, 2): (i) preconditioning of the solid-phase aliphatic-alcohol-bearing resin with 5 mL of 9 M HCl (discarded to waste); (ii) a loading step, in which 5 mL 9 M HCl (1 mL load; 4 mL rinse) containing 237Np/233Pa is passed through the column and collected to recover 237Np parent material, while 233Pa is selectively retained via adsorption to the primary alcohol solid phase; (iii) a rinse step (30 mL 9 M HCl to remove remaining 237Np parent material); and (iv) elution of radiochemically-pure 233Pa in 30 mL of 1 M HCl. Radioactivity counting sources were prepared by standard alpha spectrometry techniques. Radiochemical purity was evaluated by alpha spectrometry using standard solid-state ion-implanted silicon detectors and HPGe gamma-ray spectrometry. Significant improvements that were observed relative to previous methods include [7, 15, 16, 24]:
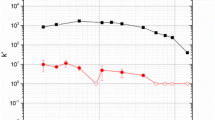
Elution curves that describe the procedure to separate 237Np and 233Pa. 1 mL fractions of the procedure were collected in a 20 mL LS vial containing 15 mL of Ecolite LS cocktail. Rapid recovery of 237Np was performed in 9 M HCl. Subsequent recovery of 233Pa was performed by passing 30 mL 1 M HCl over the column
Less waste
Previous separation protocols for the isolation of 233Pa and 237Np generally require a liquid–liquid extraction step that utilizes a 50 mL organic phase separatory-funnel-based step, with up to five subsequent-additional aqueous washes (50 mL each) to complete the purification of extracted 233Pa [16]. Our new approach results in a maximum volume of 60 mL HCl residue and no radioactive liquid organic solvent waste. We anticipate future refinements will lead to further reduction of the total acid volume required.
Improved safety and efficiency (removal of HF and sulfuric acid; H2SO4)
All previous approaches to 237Np/233Pa separation (to our knowledge) include the use of HF or H2SO4 for separation and isolation of 233Pa and 237Np [16, 24]—and prevention of Pa hydrolysis through the separation procedure [31]. The use of these reagents not only adds significantly to potentially hazardous radioactive mixed waste, but also requires additional steps to remove F− and \( {\text{SO}}_{4}^{ 2- } \) for subsequent source preparation and radioactivity measurements [6, 15]. Our new approach eliminates the need for F− and \( {\text{SO}}_{4}^{ 2- } \). At the tracer concentrations used for this study, we observed no evidence of irreversible Pa hydrolysis.
Improved efficiency
Column chromatography as a whole has greatly simplified and reduced the hands-on time for chemical separations, relative to liquid–liquid extractions. While previous liquid–liquid extraction procedures require approximately 5 h of technician-hands-on attention, our new approach with this extraction chromatographic resin containing 1-octanol as the extractant allows separation and isolation of radiochemically-pure fractions of 237Np and 233Pa in less than an hour.
Isolation of neptunium
The Np fraction was isolated (n = 3) in outstanding purity with excellent recovery for future preparations of 233Pa tracer solution. This fraction is collected in 9 M HCl, which is convenient for future Np storage and can be easily converted to other desired chemical matrices for other applications [32]. While previous studies hypothesized that primary alcohols may represent an adsorption nucleation site for Np(VI), this study finds no evidence of Np retention on the column, resulting in an effective radiochemical separation from Pa(V) in a system containing the primary alcohol, 1-octanol, thus suggesting that Np is maintained in the pentavalent oxidation state. Evaluation by gamma spectrometry demonstrated excellent radiochemical purity with the absence of 233Pa gamma-ray emission peaks (311.9, 300.1, 340.5 keV and X-rays; kαPa and kβPa; Fig. 3). Based on these analyses, the radiochemical purity of 237Np was calculated to be 99 ± 2 %, where the uncertainty is primarily attributed to low counts in the 233Pa ROI’s with activities at or below the detection limit (~2.4 mBq). Importantly, the radiometric purity of a 237Np source begins to decrease rapidly (~2 % per day) with the ingrowth of 233Pa, daughter of 237Np. From an elemental mass perspective, although the radiometric purity of the 237Np solution decreases due to daughter ingrowth, the atomic (mass) purity will remain essentially 100 % 237Np, due to the short half-life of 233Pa. However, routine removal of 233Pa is an important consideration for Np research because it will minimize overall radiation exposure to the researcher by reducing the gross beta activity and removing relatively high-energy 233Pa gamma rays.
High-purity germanium gamma-ray spectra of the a stock solution, b 237Np fraction, c and 233Pa fraction analyzed following the separation, to assess the purity of 237Np and the yield of 233Pa. The purity of the 237Np was assessed in the regions 300.1, 311.9, and 340.5 keV and was determined to be 99 ± 2 %. The yield of 233Pa was determined from the 311.9 keV region and was determined to be 99 ± 1 %. All peaks on the spectra were identified and related to 237Np and 233Pa gamma and X-ray emission except for two peaks, which were associated with natural ambient background emissions (i.e. found in blank counts of a standard of the same geometry and count time)
The radiochemical yield of 237Np was determined by alpha spectrometry using cerium hydroxide micro-precipitated sources. We observed that quantitative co-precipitation of Np was ensured through redox control by addition of H2O2 due to reduction of the soluble Np(V) to the insoluble Np(IV) form [33, 34]. While in aqueous solution Np(V) is the most stable oxidation state, it will not be incorporated into the cerium hydroxide microprecipitation, so Np(V) must be reduced. Previous research demonstrates that Np(V) can be reduced to Np(IV) with the addition of excess H2O2 [2, 35]. Thus, excellent radiochemical yields for 237Np (92 % ± 3) were achieved based analysis of alpha spectra (4.7 MeV peak; Fig. 4; Tables 1, 2) and losses could be attributed the micro-precipitation step rather than column elution step.
Alpha spectra obtained from cerium hydroxide precipitated sources used to determine the yield of 237Np and purity of 233Pa. a 237Np alpha spectra which was counted for 24 h, the radiochemical recovery was 92 ± 3 %, as seen in the region of interest at 4.78 MeV. b Alpha spectra of the 233Pa counted for 100 h. The integrated count rate of 237Np region of interest (4.78 MeV) results in a radiochemical purity of 100 ± 0.2 % for 233Pa. A peak identified as 238Pu (5.49 MeV) is expected according to the certificate provided by the manufacturer of the 237Np standard and represents approximately 0.5 mBq
Isolation of protactinium
Following the isolation of the 237Np parent material in 9 M HCl, in which 233Pa is strongly retained on the column, 233Pa (n = 3) was recovered with near quantitative radiochemical yield by elution with 30 mL of 1 M HCl (Fig. 1). While the behavior of Pa under these conditions afforded excellent separation from Np, the chemical form of the extraction Pa species into 1-octanol is not well understood [17]. Previous investigations have identified the predominant species of Pa and Np under the conditions of our separations protocol as existing in the pentavalent oxidation state, and it has been generally believed that the behavior of these elements is similar [36]. Although it is well understood that Np(V) in aqueous conditions forms the linear dioxo-neptunyl cation, \( {\text{NPO}}_{2}^{ + } \) [2, 37], less is known about the molecular features of the Pa complex in solution. It was hypothesized, and recently demonstrated, that Pa forms a linear mono oxo-protactinyl moiety [31, 37, 38]. The extracted species of Pa, in concentrations of [HCl] > 4 M, are suspected to be \( {\text{PaCl}}_{7}^{3- } \), \( {\text{PaCl}}_{6}^{2- } \), \( {\text{PaOCl}}_{6}^{3- } \), \( {\text{Pa(OH)OCl}}_{6}^{2- } \), \( {\text{PaOCl}}_{5}^{2- } \), but no dominant species has been empirically agreed upon [2, 31, 39, 40]. To quantitatively desorb Pa from the stationary phase, the [HCl] is diluted to 1 M, and the resulting Pa species is likely to be PaOOH2+ [2].
In addition to the lack of information regarding the dominate Pa(V) species, the exact mechanism for the extraction is unclear. Previous studies hypothesized that the interactions between Pa and the extractant are mainly electrostatic, as the highly acidic environment forms a protonated alcohol, [HROH+]1-2, and anionic Pa complex [13]. However, electrostatic interactions alone do not sufficiently describe the selectivity to Pa over Np, because Np is also expected to form anionic complexes in 9 M HCl [2]. Future studies in our laboratory seek to identify the specific differences in speciation that allow for the selective extraction of Pa over Np with 1-octanol.
The radiochemical yield for 233Pa was 99 ± 1 %, determined by HPGe gamma spectroscopy based on the gamma emission at 311.9 keV (Fig. 3). Each fraction was decay corrected based on the time elapsed from Pa elution to start of gamma spectral collection. Radiochemical purity of 233Pa was determined from a 100-h count of a cerium hydroxide micro-precipitated source by alpha spectrometry. Radiochemical purity of the 233Pa sources was determined to be 100 ± 0.2 %. Nearly quantitative isolation from 237Np parent material was achieved, with ~5 mBq (count rate <0.002 counts/s) of the 14.5 Bq 237Np added (0.03 %) to the solution were identified by alpha particle emission on the 233Pa (Fig. 4; Table 2). While this separation factor approaches the limitations of extraction chromatographic resins, additional steps can be taken to further purify 233Pa if necessary. These include: (1) passing the solution through the 1-octanol column additional times until the desired purity is achieved; (2) application of the cerium hydroxide micro-precipitation step without the addition of an oxidizing agent, which would exclude precipitation of 237Np, which does not form a cerium hydroxide micro-precipitation as Np(V); and (3) the use of multiple-smaller-increment elution volumes to improve the overall removal of Np (i.e. 6 rinses of 5 mL 9 M HCl vs. 1 rinse of 30 mL).
Conclusions
A novel approach to efficient separation and isolation of Np and Pa into radiochemically-pure fractions, using a new chromatographic resin material has been described. The approach takes advantage of highly-selective adsorption of Pa to primary-aliphatic alcohols in HCl media using a solid-phase chromatography resin-based material with 1-octanol as the extractant. The material can be produced easily in-house (at low cost) and our observations suggest that the material shelf-life exceeds 1 year without apparent degradation (stored at room temperature under typical laboratory conditions). The new approach significantly reduces waste (e.g. acid, organic, radioactive, and mixed) produced during the separation and isolation of 233Pa and 237Np and eliminates the need for HF and H2SO4. The method reproducibly (n = 3) isolated 233Pa tracer with a yield of 99 ± 1 % (radiochemical purity 100 %) and enabled chemical recovery of 237Np parent material of 92 ± 3 % (purity >99 %) for future 233Pa tracer preparations. It is anticipated that the procedure could be adjusted to meet more stringent criteria for purity and yield of Pa and Np.
References
Emsley J (2003) Nature’s building blocks: an A to Z guide to the elements. Oxford University Press, Oxford
Morss LR, Edelstein NM, Fuger J (2010) The chemistry of the actinide and transactinide elements, vol 1. Springer, Dordrecht
Peate DW, Hawkesworth CJ (2005) Rev Geophys. doi:10.1029/2004RG000154
Negre C, Thomas AL, Mas JL, Garcia-Orellana J, Henderson GM, Masque P, Zahn R (2009) Anal Chem 81(5):1914–1919
Bourdon B, Turner S, Henderson GM, Lundstrom CC (2003) Rev Miner Geochem 52:1–21
Regelous M, Turner SP, Elliott TR, Rostami K, Hawkesworth CJ (2004) Anal Chem 76(13):3584–3589
Eppich GR, Williams RW, Gaffney AM, Schorzman KC (2013) J Anal Atom Spectrom 28(5):666–674
Morgenstern A, Apostolidis C, Mayer K (2002) Anal Chem 74(21):5513–5516
Keegan RP, Gehrke RJ (2003) Appl Radiat Isot 59(2–3):137–143
Eskandari Nasab M (2014) Fuel 116:595–600
Wilson RE (2012) Nat Chem 4(7):586
De Sio SM, Wilson RE (2014) Inorg Chem 53(3):1750–1755
Kumari N, Pathak PN, Prabhu DR, Manchanda VK (2012) Desalin Water Treat 38(1–3):46–51
Uhlir J (2005) In: Nakajima T, Groult H (eds) Fluorinated materials for energy conversion. Elsevier, Amsterdam
Pickett DA, Murrell MT, Williams RW (1994) Anal Chem 66(7):1044–1049
Sill CW (1966) Anal Chem 38(11):1458
Kirby HW (1959) National Academy of Sciences National Research Council, Nuclear Series
Turner DR, Pabalan RT, Bertetti FP (1998) Clay Clay Miner 46(3):256–269
Zhao P, Tinnacher RM, Zavarin M, Kersting AB (2014) J Environ Radioact 137:163–172
Nakata K, Fukuda T, Nagasaki S, Tanaka S, Suzuki A, Tanaka T, Muraoka S (1999) Czech J Phys 49:159–166
Bubernak J, Lew MS, Matlack GM (1969) Anal Chim Acta 48(2):233–241
Hull CD, Burnett WC, Cable P, Yeh CC (1992) U-series dating using extraction chromatography; U, Pa in geological using TRU resin and alpha-spec. Abstract HU192. 38th annual conference on bioassay, analytical and environmental radiochemistry, Santa Fe, NM
Tylenda CA, Jones D, Ingerman L, Sage G, Chappell L (2001) Agency for Toxic Substances and Disease Registery, Atlanta
Burnett WC, Yeh CC (1995) Radioact Radiochem 6(4):22–32
National Nuclear Data Center (2015) Information extracted from the NuDat 2 database. http://www.nndc.bnl.gov/nudat2/
Currie LA (1968) Anal Chem 40(3):586–593
Horwitz EP, McAlister DR, Dietz ML (2006) Separ Sci Technol 41(10):2163–2182
Knight AW, Eitrheim ES, Nelson AW, Nelson S, Schultz MK (2014) J Environ Radioact 134:66–74
Nelson AW, May D, Knight AW, Eitrheim ES, Mehrhoff M, Shannon R, Litman R, Schultz MK (2014) Environ Sci Technol Lett 1(1):204–208
Inn KGW, Hall E, Woodward JT IV, Stewart B, Pollanen R, Selvig L, Turner S, Outola I, Nour S, Kurosaki H, LaRosa J, Schultz MK, Lin Z, Yu Z, McMahon CJ (2008) J Radioanal Nucl Chem 276(2):385–390
Le Naour C, Trubert D, Di Giandomenico MV, Fillaux C, Den Auwer C, Moisy P, Hennig C (2005) Inorg Chem 44(25):9542–9546
Mendes M, Aupiais J, Jutier C, Pointurier F (2013) Anal Chim Acta 780:110–116
Tananaev IG, Dzyubenko VI (1989) Soviet Radiochem (Eng Transl) 30(6):797–800
Burney GA, Harbour RM (1974) The radiochemistry of neptunium, National Academy of Sciences National Resource Council, Nuclear Series
Johnsen AM (2008) Neptunium dioxide precipiation kinetics in aqeous systems, PhD Dissertation. University of California, Berkley
Siboulet B, Marsden CJ, Vitorge P (2008) New J Chem 32(12):2080–2094
Toraishi T, Tsuneda T, Tanaka SJ (2006) Phys Chem 110(49):13303–13309
Mendes M, Leguay S, Le Naour C, Hamadi S, Roques J, Moisy P, Guillaumont D, Topin S, Aupiais J, Den Auwer C, Hennig C (2013) Inorg Chem 52(13):7497–7507
Guillaum R, Bouissie G, Muxart R (1968) Actin Rev 1(2):135
Scherff HL, Herrmann G (1966) Radiochim Acta 6(2):53
Acknowledgements
The authors would like to thank Phil Horwitz, Daniel McAlister, and Eichrom Technologies for the production of the resin form of 1-octanol for these experiments. This material is based upon work supported by the U.S. Department of Homeland Security under Grant Award Number, 2012-DN-130-NF0001-02. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the U.S. Department of Homeland Security.
Conflict of interest
The authors declare no competing financial interest. Interested investigators are invited to inquire with the authors for access to the new resin-based material for further research applications.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Knight, A.W., Nelson, A.W., Eitrheim, E.S. et al. A chromatographic separation of neptunium and protactinium using 1-octanol impregnated onto a solid phase support. J Radioanal Nucl Chem 307, 59–67 (2016). https://doi.org/10.1007/s10967-015-4124-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4124-3