Abstract
A procedure for the separation of technetium isotopes from bulk molybdenum was developed in nitric acid media. After irradiation of natMo foils, and dissolution in H2O2, the solution is acidified to 2 M HNO3, and anion exchange resin is used to separate technetium from molybdenum. The procedure is simple, requiring only basic laboratory equipment and, with a two-column separation, the technetium yield is high (~ 85%) with extremely high purity (< 0.1 ppm natMo). This is ideally suited for laboratory production of technetium tracer isotopes, particularly 95mTc.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Technetium chemistry is extremely important for some of the most critical areas of modern radiochemical research: the environmental impacts of the nuclear fuel cycle and radiopharmaceuticals. As a long-lived, high-yield fission product, environmental studies of 99Tc are extremely important for monitoring the release of radionuclides from spent nuclear fuel, particular from reprocessing facilities [1,2,3,4]. Technetium-99m, the short-lived daughter of 99Mo, is an extremely important radiopharmaceutical isotope and is used millions of times a year to provide diagnostic information for medical exams [2,3,4,5,6,7].
Tracer isotope studies can be a valuable way to study the chemistry of technetium to better inform research applications in these areas. For these studies, 95mTc is often more useful than either 99m,gTc due to its more favorable half-life and gamma-ray emissions [8, 9]. It can also be used as a yield tracer for studies with 99Tc, as no stable isotope of technetium is available [3]. Both isomers of 95Tc are readily produced via proton or deuteron irradiation of natMo. While carrier-free molybdenum/technetium separations are well-characterized, and 99Mo/99mTc isotope generators are routinely used worldwide in hospitals and laboratories [5, 10], the separation of technetium isotopes from bulk molybdenum targets is more challenging [10]. There is active research in this area [5, 7, 11, 12], largely to ensure the continued availability of 99mTc for nuclear medicine, but general methods are not well established and new research in this area often involves complex chemistry designed to meet the stringent purity requirements for medical applications. A number of proprietary resins are being developed to respond to this need, including AnaLig®Tc-02 resin from IBC Advanced Technologies Inc. and the TK series of resins (TK-201, -202, -TcScint) from Triskem International. For tracer isotope production, which is not subject to the stringent limitations imposed by biological compatibility concerns, a simple, high-yield, high purity separation without an excess of radioactive waste would be ideal.
Methods suggested in the literature for the separation of technetium from bulk molybdenum include column chromatography [1, 7, 8, 11, 13], sublimation [6, 14, 15], thermochromatography [16], and liquid–liquid extraction [8, 10, 17]. Column chromatography is often advantageous compared to other techniques as there is less waste and usually higher separation factors for a single pass separation. Columns require no specialized equipment nor handling radioactive gases (as with sublimation and thermochromatography techniques). However, many of the column separations in the literature require organic compounds, particularly ammonium thiocyanate [13] and tetrabutyloammonium bromide with dicholomethane [7, 18], that have toxicity concerns and could lead to trace organics in the final product unless specific purification steps are taken, which can be undesirable. Other column Extraction chromatography with Aliquat 336-based resins have been used for the separation of 99Tc from molybdenum as well [1, 8], though reported yields are low, likely due to peroxide damage to the resin as hydrogen peroxide is generally used to dissolve molybdenum [8]. A common procedure employs ammonium carbonate ((NH4)2CO3) to retain technetium on anion exchange resin while molybdenum is eluted [11, 19], followed by stripping of technetium with nitric acid [11] or water [19].
As the ammonium carbonate-based separations typically have high reported yields, initial studies were conducted to compare a new separation against a literature procedure [11] under the same conditions. Based on these results, a simple procedure was developed with improved purification of molybdenum from technetium in a nitric acid medium with anion exchange resin. The procedure is high yield and high purity, suitable for tracer isotope studies for a variety of radiochemical applications.
Experimental
Separations were performed with anion exchange resin (AG 1 × 8, 100–200 mesh, BioRad). Solutions of HNO3 and HCl were prepared from ULTREX II ultra-pure acids (J.T. Baker) diluted with Aristar ultra-pure water (VWR International), as necessary. The concentrated HNO3 had a concentration of 15.33 M based on the specific batch analysis performed by J.T. Baker. Ammonium carbonate (ACS reagent, Merck) and sodium hydroxide (ACS reagent, Sigma) were dissolved in Aristar ultra-pure water (VWR International) to make solutions. The anion exchange resin was prepared by washing with 1 M HCl, water, 1 M NaOH, water, 1 M HCl, water, 1 M NaOH, water (two times), ethanol, and water; it was stored in dilute HCl.
Two molybdenum foils (99.98%, 20 µm thick, Goodfellow) were irradiated at the Center for Accelerator Mass Spectrometry (CAMS) at Lawrence Livermore National Laboratory (LLNL) with 11 MeV protons using a 10-MV FN tandem Van de Graaff accelerator. The total irradiation time was 3 h with an average beam intensity of 1200 nA. Each foil was counted at the Nuclear Counting Facility at LLNL to determine the technetium isotopes and activities (listed in Table 1 along with relevant decay information). The foils were then left to decay for a sufficient period (~ 2 months) for all isotopes other than 95mTc to decay to near background. While 95gTc is short-lived (20.0 h), it is still present after the decay period because there is an equilibrium between the short-lived ground state and longer-lived metastable state, which has a minor isomeric transition (IT) decay branch (3.88%).
Foil 1 (15.94 mg) was cut approximately into quarters, and two of these were used to directly compare two different chemical procedures, one novel separation and one based on a procedure from the literature [11]. The remainder of Foil 1 and all of Foil 2 (14.68 mg) were used to test the final separation procedure. A flow chart of the sample processing based on the initial irradiated foils is shown in Fig. 1.
For the chemical procedure tests, all gamma-ray spectrometry measurements were performed with an HPGe gamma-ray detector with Ortec NIM electronics and ASPEC multi-channel analyzer; spectra were analyzed with Maestro software (Ortec). Measurements of all column fractions were relative to the load solution, and all samples were counted in the same geometry immediately after elution. Mass spectrometry measurements were performed with a Thermo Scientific iCAP quadrupole inductively coupled plasma mass spectrometer (ICP-MS). Full quantitative analysis was done with a linear calibration curve based off external standards. An internal standard was used to correct for matrix signal suppression and instrument drift. The uncertainty on the mass spectrometry measurements is two standard deviations (2SD).
Initial comparison studies
The samples Q1 and Q2 were each placed into a 5 mL tube and dissolved in hydrogen peroxide (H2O2, 30% wt, non-stabilized, Acros). The tubes were capped and heated (60 °C) during the dissolution to ensure no loss of technetium as it can be volatile in oxidizing solutions [21]. After cooling to room temperature, each solution was diluted to 2 mL (see Table 2) depending on the final concentration desired for the load solution. To Q1, 1 mL 4 M HNO3 was added and to Q2 0.5 mL 3 M (NH4)2CO3 was added based on Ref. [11]. A 20 µL aliquot was removed from each solution for mass spectrometry analysis and the samples were counted with an HPGe detector. Each sample was loaded onto a 2 mL BioRad column filled with 4.2 cm AG 1 × 8 resin and preconditioned with 8 mL of the same solution as the load solution. Fractions were collected in 2 mL increments; the elution details are given in Table 2.
Full separation procedure
Once the initial separations were completed and the results compared, the other samples from the irradiated molybdenum foils (Sample 1 and Sample 2; see Fig. 1) were prepared for separation. Each sample was placed in a 5 mL tube with 1 mL H2O2. The tubes were capped and heated at 60 °C until the foil dissolved (~ 1 h). The solution was allowed to cool, then 1 mL 4 M HNO3 was added. A 20 µL aliquot of each solution was removed for mass spectrometry analysis. Anion exchange columns were prepared identically to the previous ones (4.2 cm AG 1 × 8 resin) and pre-conditioned with 8 mL 2 M HNO3 immediately before elution. Each dissolved molybdenum sample was loaded onto a column; the load fraction was collected, followed by 10 mL 2 M HNO3, then 10 mL conc. HNO3. All fractions were 2 mL. For each column, the fraction with the majority of the activity (fraction 7) was diluted to ~ 15 mL to result in a final concentration of 2 M HNO3. This was loaded onto a second anion exchange column prepared and conditioned identically to the first. The load solution was collected, followed by elution with 10 mL 2 M HNO3 and 10 mL conc. HNO3, all collected in 2 mL fractions. After each fraction was counted with gamma spectroscopy, as before, a 20 µL aliquot was removed from the fraction with the highest activity (fraction 7) for mass spectrometry analysis.
Results and discussion
Initial comparison studies
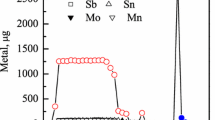
The results from the initial columns are shown in Fig. 2. These columns compared a new procedure (Fig. 2a) with a procedure from the literature (Fig. 2b). The elution pattern is fairly similar for each. Technetium-95 is well retained from the load solution and stripped with a high yield in concentrated HNO3. In the new procedure (Fig. 2a), molybdenum is eluted from the column with only 2 M HNO3, which is immediately followed by elution of technetium with conc. HNO3. The procedure based on the literature elutes molybdenum with (NH4)2CO3 to followed by a rinse with water, likely to ensure the column is not basic when acid is added in the next step [11]. While Ref. [11] uses 4 M HNO3 to elute technetium, after no 95mTc was detected after eluting the column in Fig. 2b with 2 bed volumes of 4 M HNO3, the eluant was switched to concentrated HNO3 to enable a high yield in a small number of bed volumes. For each column, all of the technetium fractions were combined after elution and an aliquot was removed to determine the final concentration of molybdenum with mass spectrometry. The mass spectrometry data comparing the initial and final concentrations of molybdenum in each sample are shown in Table 3 along with the total yield of 95mTc for each column.
Comparison of 95mTc elution on AG 1 × 8 resin from solutions of dissolved molybdenum in HNO3 and (NH4)2CO3 solutions. The load solutions and all fractions were 2 mL. The initial concentration of molybdenum was ~ 220 µg/g solution. The elution of molybdenum is not plotted as there were no gamma-ray emissions from a molybdenum isotope. Most error bars are smaller than the data points; lines are to guide the eye
The yield from these separations is similar (within error). However, the reduction in molybdenum mass is ~ 4 times better for Q1 than for Q2. In the separation used for Q1, for which both the load solution and the molybdenum elution used a 2 M HNO3 solution, the pertechnetate anion (TcO4−) is retained in 2 M HNO3, while molybdenum can be eluted as a cation (MoO22+) [22]. In the separation used for Q2, with a (NH4)2CO3 solution used for both the load solution and elution of molybdenum, molybdenum forms a neutral species (NH4)2MoO4, which elutes, while TcO4− is retained. Technetium is eluted from both columns as the concentration of HNO3 increases. The improvement in molybdenum stripping in 2 M HNO3 is difficult to fully describe as neither the cationic MoO22+ nor the neutral (NH4)2MoO4 should have any affinity for the resin. However, as the results for Q1 were clearly better than for Q2, a procedure to purify technetium tracer isotopes from the remaining foil samples was developed based on the conditions used for the Q1 separation.
Full separation procedure
The larger foils samples, Sample 1 (the other half of foil 1) and Sample 2 (all of foil 2), were dissolved and eluted on anion exchange columns identically to Sample A. Despite the varying masses of molybdenum, the elution profiles were extremely similar as shown in Fig. 3.
Elution of 95mTc on AG 1 × 8 resin from solutions of dissolved molybdenum in 2 M HNO3. The load solutions and all fractions were 2 mL. The initial concentration of molybdenum was 3.4 mg/g solution (Sample 1) and 7.3 mg/g solution (Sample 2). The elution of molybdenum is not plotted as there was no gamma emission from a molybdenum isotope. Most error bars are smaller than the data points; lines are to guide the eye
To further reduce the amount of molybdenum remaining in the samples, the fraction with the most technetium from each column (fraction 7) was diluted to 2 M HNO3 and eluted again on an identical anion exchange column. The results from this second separation are shown in Fig. 4. The load fraction (~ 16 mL) was much larger than the previous columns, but the column yield was extremely high (~ 100%) for 95mTc, likely due to the reduced molybdenum mass. The yields for each column, as well as the total yields, are shown in Table 4. The initial and final concentrations of molybdenum for each sample are shown in Table 5.
After the two columns, the concentration of molybdenum is only slightly elevated from the background level, which, in ultra-pure acid, is 0.0064 ± 0.001 µg/g. Direct purity comparisons to other procedures in the literature can be difficult as often an absolute concentration is not reported, and the initial mass varies between experiments. For example, Refs. [11] and [18] both report a final concentration of molybdenum as < 10 ppm, but Ref. [11] has an initial concentration of molybdenum similar to this work, while Ref. [18] is two orders of magnitude higher. Reference [15] separated 99mTc from small molybdenum targets (~ 10 mg) with < 1 µg molybdenum remaining in the technetium product, which would indicate about 0.01% of the molybdenum mass remaining in the final product, despite a multi-step chemical procedure including a precipitation, sublimation and a column. This work used smaller targets (~ 7 mg maximum) but achieved a larger reduction of molybdenum with a far simpler chemical procedure. Other works, e.g. [7] and [10], report the purity in terms of the radiopurity by detected 99Mo in activated, bulk 100Mo, which is not easily compared to a mass concentration.
In terms of yield, the results from this work improve upon Ref. [8] and are comparable to or better than Ref. [7], which achieves yields of ~ 85% with a technetium specific resin (AnaLig Tc-02), but lower yields with 1 × 8 resin (~ 75%), as used on this work. Reference [12] also employs AnaLig Tc-02 and with a 3-column procedure is able to achieve a high technetium yield (~ 90%) with minimal molybdenum contamination in the final product (< 0.04 ppm) despite a large starting mass (250 mg). This separation may be preferrable if the expense of acquiring the AnaLig resin is not prohibitory. The procedure used in this work is ideally suited to rapid, small scale laboratory production of technetium tracer isotopes. The yield and purity for the two-column procedure are high, and the separation can be performed rapidly (~ 3 h) with minimal equipment, low costs and no organic waste.
Conclusions
Tracer isotopes are useful to study the chemistry of technetium for a variety of applications including the environmental effects of the nuclear fuel cycle and radiopharmaceuticals. While there is a considerable amount of active research into separations of 99mTc from molybdenum for the production of 99mTc for nuclear medicine applications, there are fewer separations suitable for basic laboratory production of tracer isotopes, which do not need to meet stringent requirements based on biological compatibility. To assess the development of a new, simple procedure for the separation of technetium tracer isotopes from bulk molybdenum, a comparison study was performed initially. The separation developed in this work was compared under identical conditions to a procedure well-known in the literature and the yield was comparable, while the purity was improved.
Therefore, a two-column procedure was developed to enable high-yield, high purity separations of technetium from irradiated molybdenum foils. The purity is extremely high with the concentration of molybdenum reduced to near background levels with a high yield (~ 85%). The procedure uses only basic equipment and does not involve radioactive gasses or produce organic waste, as in sublimation separations and liquid–liquid extraction separations. It improves upon other tracer isotope separation procedures with 1 × 8 anion exchange resin in terms of the yield and produces technetium suitable for a variety of tracer isotope applications.
References
Shi K, Hou X, Roos P, Wu W (2012) Stability of technetium and decontamination from ruthenium and molybdenum in determination of 99Tc in environmental solid samples by ICPMS. Anal Chem 84(4):2009–2016
Leonard K, Mccubbin D, Mcdonald P, Service M, Bonfield R, Conney S (2004) Accumulation of technetium-99 in the Irish Sea. Sci Total Environ 322(13):255–270
Bergquist BA, Marchetti AA, Martinelli RE, McAninch JE, Nimz GJ, Proctor ID, Southon JR, Vogel JS (2000) Technetium measurements by accelerator mass spectrometry at LLNL. NIM-B 172(1–4):328–332
Ustynyuk YA, Zhokhova NI, Sizova ZA, Nenajdenko VG (2024) Recent progress in separation of technetium-99 from spent nuclear fuel and radioactive waste. Challenges and prospects. Coord Chem Rev 508:215759
Dash A, Knapp F Jr, Pillai M (2013) 99Mo/99mTc separation: an assessment of technology options. Nucl Med Biol 40(2):167–176
Christian J, Petti D, Kirkham R, Bennett R (2000) Advances in sublimation separation of technetium from low-specific-activity molybdenum-99. Ind Eng Chem Res 39(9):3157–3168
Wojdowska W, Pawlak D, Parus J, Mikołajczak R (2015) Studies on the separation of 99mTc from large excess of molybdenum. Nucl Med Rev 18(2):65–69
Fikrle M, Kucera J, Sebesta F (2010) Preparation of 95mTc radiotracer. J Radioanal Nucl Chem 286:661–663
Hirano S, Matsuba M (1993) Concentrations of technetium-99 in marine algae and seawater. Radiochim Acta 63(s1):79–82
Martini P, Boschi A, Cicoria G, Uccelli L, Pasquali M, Duatti A, Pupillo G, Marengo M, Loriggiola M, Esposito J (2016) A solvent-extraction module for cyclotron production of high-purity technetium-99m. Appl Radiat Isot 118:302–307
Das MK, Madhusmita CS, Das SS, Barua L, Neyar MA, Kumar U, De A (2016) Production and separation of 99mTc from cyclotron irradiated 100/naturalMo targets: a new automated module for separation of 99mTc from molybdenum targets. J Radioanal Nucl Chem 310:423–432
Koźmiński P, Gumiela M, Walczak R, Wawrowicz K, Bilewicz A (2021) A semi-automated module for the separation and purification of 99mTc from simulated molybdenum target. J Radioanal Nucl Chem 328:1217–1224
Hall N, Johns D (1953) The separation of technetium from molybdenum, cobalt and silver. J Am Chem Soc 75(23):5787–5791
Boyd G, Larson Q, Motta E (1960) Isolation of milligram quantities of long-lived technetium from neutron irradiated molybdenum. J Am Chem Soc 82(4):809–815
Sabelnikov AV, Maslov OD, Molokanova LG, Gustova MV, Dmitriev SN (2006) Preparation of 99Mo and 99mTc by 100Mo(γ, n) photonuclear reaction on an electron accelerator, MT-25 microtron. Radiochemistry 48:191–194
Rösch F, Novgorodov AF, Qaim SM (1994) Thermochromatographic separation of 94mTc from enriched molybdenum targets and its large scale production for nuclear medical application. Radiochim Acta 64:113–120
Ejaz M, Mamoon A (1988) Studies on the solvent extraction of technetium from thiocyanate solutions in mineral acids. J Radioanal Nucl Chem 125:419–430
Chattopadhyay S, Das S, Das M, Goomer N (2008) Recovery of 99mTc from Na2[99Mo]MoO4 solution obtained from reactor-produced (n, γ)99Mo using a tiny Dowex-1 column in tandem with a small alumina column. Appl Radiat Isot 66(12):1814–1817
Gagnon K, Wilson J, Holt C, Abrams D, McEwan A, Mitlin D, McQuarrie S (2012) Cyclotron production of 99mTc: Recycling of enriched 100Mo metal targets. Appl Radiat Isot 70(8):1685–1690
National Nuclear Data Center (2013) Brookhaven National Laboratory. https://www.nndc.bnl.gov/nudat2/indx_dec.jsp. Accessed 11 Nov 2023
Schwochau K (2005) The analytical chemistry of technetium. Inorgan Chem Top Curr Chem 96:109–147
Tkac P, Paulenova A (2008) Speciation of molybdenum (VI) In aqueous and organic phases of selected extraction systems. Sep Sci Technol 43(9–10):2641–2657
Acknowledgements
The authors thank the staff of the CAMS facility at LLNL, particularly Scott Tumey and John Wilkinson, for providing beam time and expertise to produce the technetium isotopes. This study was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest or competing interest relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kmak, K.N., Despotopulos, J.D. & Kerlin, W.M. Simple separation of technetium from molybdenum for tracer isotope production. J Radioanal Nucl Chem 333, 2759–2766 (2024). https://doi.org/10.1007/s10967-024-09506-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-024-09506-6