Abstract
With the aim of developing a new tumor imaging agent, the IFLLWQR (Ile-Phe-Leu–Leu-Trp-Glu-Arg, IF7) was conjugated with 1, 4, 7-triazacyclononane-N, N’, N’’-triacetic acid (NOTA) and labeled with 68Ga. In the optimal conditions, the whole radio-synthesis was accomplished within 20 min. The decay-corrected radiochemical yield was more than 92 %. The radiochemical purity of 68Ga-NOTA-IF7 was more than 95 %. MicroPET studies showed a high tumor uptake at 15 min post-injection with 7.52 ± 0.16 %ID/g. The ratios of tumor to muscle uptake were 20.61 ± 0.31, 13.14 ± 0.21, 2.39 ± 0.12 at 15 min, 30 min and 60 min post-injection, respectively. 68Ga-NOTA-IF7 is a promising radiopharmaceutical for tumor imaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tumor angiogenesis is the process of new blood vessel formation necessary for tumor growth and metastasis [1]. Anxa 1 is a highly specific surface marker of tumor vasculature. The carbohydrate ligand-mimicking I-peptide can specifically target Anxa 1 in tumor [2, 3]. In recent years, major efforts and progress have been made in the development of carbohydrate mimetic peptide. Previous study of IF7 peptide conjugated with fluorescent Alexa 488 (A488) in tumor-bearing-mice showed that IF7 had an excellent targeting property to Anxa1, a specific marker on the tumor endothelium [4].
Positron emission tomography (PET) is a modern medical imaging technique that provides useful information about physiological and biochemical disorders on the molecular level [5]. Radiolabeled peptides and proteins have been the subject of intense research efforts for targeted diagnostic imaging and radio-therapy in nuclear medicine over the last decade years. Peptides and proteins play an important role as key regulators of cell growth and cellular function in living organisms [6]. Due to the increasing relevance of peptides for molecular imaging purposes, many reports have been published on the labeling of peptides with the short-lived positron emitter. The importance of 68Ga for clinical PET has increased recently [7]. 68Ga (t 1/2 = 68 min) is a very attractive radionuclide for PET, as it can be produced from a 68Ge/68Ga generator (68Ge, t 1/2 = 270.8 day), allowing easy routine manufacture compared with other positron radionuclide [6, 8, 9].
NOTA (1,4,7-triazacyclononane-N, N′, N′′-triacetic acid) is a chelator for labeling of targeting agents with a number of radiometals. Derivatives of NOTA are very promising chelators for radio-labeling of targeting proteins and peptides. The radionuclide 68Ga can be introduced into proteins and peptides using NOTA as a chelator. NOTA can provide stable complexes with gallium [10]. Site-specific labeling is important to provide chemically uniform radio-conjugates with well-defined in vivo properties. For short peptides, such site-specificity is easy to achieve by incorporation of the chelator during peptide synthesis.
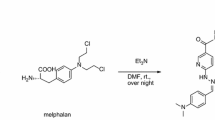
In this report, we conjugated IF7 with NOTA to produce a NOTA-IF7 conjugate. We then labeled this conjugate with 68Ga and tested the feasibility of 68Ga-NOTA-IF7 (Fig. 1) for the MicroPET imaging of Anxa1 expressed on the surface of tumor vasculature in mice bearing tumor xenografts.
Materials and methods
The p-SCN-Bn-NOTA was purchased from Macrocyclics (Dallas, TX) and IF7 was obtained from Shanghai Bootech BioScience & Technology Co., Ltd. 68Ga was obtained from a 68Ge/68Ga generator (ITG isotope technologies Garching GmbH, Germany) eluted with 0.1 N HCl. All of the other chemicals were of analytical grade and without further purification. Institute of Cancer Research (ICR) mice (weighting 18–20 g) were supplied by Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). U118 cells were kindly gifted from Professor Xiaohong Guan (Nanjing Medical University, China).
A Waters high-performance liquid chromatography (HPLC) system with a Waters 2998 photodiode array detector (PDA) using a preparative C18 HPLC column (Xbridge C18 5 μm, 250 × 19 mm, Waters) was used for peptide conjugate purification. The analytical HPLC system included a binary HPLC pump (Waters 1525, USA), a UV detector (Waters 2487, USA) and a flow scintillation analyzer (Radiomatic 610TR, Perkin-Elmer, USA). The Reversed-phase C18 column (4.6 × 250 mm, 5 μm particle size, Jiangsu Hanbon Science & Technology Co., Ltd) was eluted at a flow rate of 1 mL/min with the following buffer system: buffer A (0.1 % v/v trifluoroacetic acid in H2O); buffer B (0.1 % v/v trifluoroacetic acid in acetonitrile); a gradient of 95 % buffer A at 0–2 min to 35 % buffer A at 35 min. Mass spectra were obtained with a Waters LC–MS system.
Preparation of NOTA-IF7
NOTA-IF7 was synthesized by p-SCN-Bn-NOTA and IF7 under the reaction conditions described by the previous paper [1]. Briefly, 4.8 mg (8.6 μmol) of p-SCN-Bn-NOTA in 50 μL of dimethyl sulfoxide (DMSO) was added to a 5 mL glass vial containing 7.0 mg (7.2 μmol) of IF7 and 20 μL of diisopropylethylamine in 0.3 mL N,N-dimethylforma-mide (DMF). After 1 h, the reaction was ended with 1 mL water. The reaction mixture was purified with a preparative HPLC running a linear gradient starting from 95 % A (0.1 % TFA in water) and 5 % B (0.1 % TFA in acetonitrile) for 2 min and decreasing to 35 % A at 22 min with a flow rate of 20 mL/min. The fractions containing the desired product (52 %) were collected and lyophilized to give 6.1 mg of white powder. The purity of the product was >95 % by analytical HPLC (t R = 15.3 min). Matrix assisted laser desorption/ionization (MALDI) time-of-flight (TOF) mass spectrometry (MS) measured, [MH]+ = 1425.62 (m/z), calc: 1425.70 (C69H100N16O15S).
Preparation of 68Ga-NOTA-IF7
The fresh 68Ga activity was eluted from the 68Ge/68Ga generator with 0.1 M HCl at 0.5 mL per fraction into the 1.5 mL polypropylene tubes. The fraction containing the most radioactivity (115 MBq) was added to 0.45 mL of 1 M HEPES solution (pH 5.3) and 15 μg NOTA-IF7 in 5 μL of 0.2 M pH 4 sodium acetate buffer. The mixture was heated at 100 °C for 10 min. After cooling, a separate procedure was performed without HPLC purification. The reaction mixture was diluted with 15 mL deionized water and then loaded into a Bond Elut C18 column (Agilent Technologies). The cartridge was washed again with 15 mL water. Then the desired labeled 7-mer peptide was diluted with 0.4 mL 10 mM HCl in ethanol and passed through a 0.22 μm Millipore filter into a sterile multidose vial. The purified product was analyzed with analytical HPLC for their radiochemical purity. The retention time for 68Ga-NOTA-IF7 was 17.7 min (Fig. 2).
Biodistribution study of 68Ga-NOTA-IF7
Biodistribution study of 68Ga-NOTA-IF7 was carried out in normal mice. 68Ga-NOTA-IF7 (200 μL, about 0.74 MBq) was injected through the tail vein. At selected time points (15, 30 and 60 min), mice (n = 6 at each time point) were sacrificed. Major organs and tissues were collected and weighed. The radioactivity in these tissues was measured using a gamma counter (1470 Automatic Gamma Counter, Perkin-Elmer, USA). The results were presented as the percentage injected dose per gram of tissue (% ID/g). For each mouse, the radioactivity of the tissue samples was calibrated against a known aliquot of the injected activity. The mean uptake (%ID/g) for each group of animals was calculated with standard deviations.
Cell binding assay
The in vitro binding affinity of NOTA-IF7 was measured via displacement cell binding assays using 68Ga-NOTA-IF7 as the radio ligand. Experiments were performed on following a previously described method [11]. 68Ga-NOTA-IF7 100 μL (about 1 × 106 cpm/mL, diluted with binding buffer pH 7.4) with 100 μL of different concentrations of competitive ligand, NOTA-IF7, which was prepared in our laboratory previously, added was mixed with 100 μL U118 cell (about 2 × 106/mL). The mixture was incubated at 37 °C for 1 h. Centrifuging at 4,000 rpm was performed for 15 min, and the precipitation was collected for gamma counting. Each sample was tested in five parallel tubes.
Preparation of animal tumor models
Four to six weeks nude mice were used for the tumor engraftment. All animal experiments were carried out in compliance with the national laws related to the conduct of animal experiments. The U118 tumor model was developed in nude mice by injection of 3 × 106 cells in their left oxters. The mice underwent small animal PET studies when the tumor was inoculated 3–4 weeks. The tumor size was about 8 × 10 mm.
MicroPET imaging and analysis
MicroPET scans and image analysis were performed using an Inveon microPET scanner (Siemens Medical Solutions). About 3.7 MBq 68Ga-NOTA-IF7 was administered via tail vein injection under isoflurane anesthesia. Five-minute static PET images were acquired at 15, 30, and 60 min postinjection. The microPET images were reconstructed by 3-dimensional ordered-subsets expectation maximization (OSEM) algorithm, using Inveon Acquisition Workplace software (version 1.4; Siemens Preclinical Solutions).
For each scan, regions of interest (ROIs) were drawn using vendor software (ASI Pro 6.7.1.1) on decay-corrected whole-body images. The radioactivity concentrations (accumulation) within the tumors and muscle were obtained from mean pixel values within the multiple ROI volume. These values were then divided by the administered activity to obtain (assuming a tissue density of 1 g/mL) an image-ROI-derived percent injected dose per gram (%ID/g).
Results
NOTA-IF7 synthesis
The IFLLWQR peptide is designated as IF7. S-2-(4-Isothiocyanatobenzyl)-1, 4, 7-triazacyclononane-1, 4, 7-triacetic acid is designated as p-SCN-Bn-NOTA. The IF7 conjugated with p-SCN-Bn-NOTA is designated as NOTA-IF7. 68Gallium NOTA-IF7 complex is designated as 68Ga-NOTA-IF7. The structure of 68Ga-NOTA-IF7 is shown in Fig. 1.
NOTA-IF7 was prepared at mg scale that can be lyophilized and weighed for further use. Chemical purities of these compound was >97 % determined by analytical HPLC analysis. The identity of nonradioactive compounds was determined by MALDI-TOF, which observed m/z ions that matched their calculated molecular weights.
Radiolabelling of 68Ga-NOTA-IF7
To further simplify the labeling procedure, the solid-phase extraction method was used to prepare 68Ga-NOTA-IF7. The tracer could be prepared in about 20 min. Without the HPLC purification, the specific activity was determined by the amount of peptide used, assuming the complete recovery of peptide and the amount of radioactivity used. The decay-corrected radiochemical yield was more than 92 %. The radiochemical purity of 68Ga-NOTA-IF7 was more than 95 %. In our preparation of this tracer, 10 nmol of NOTA-IF7 was used to make 68Ga-NOTA-IF7. The specific activity of 68Ga-NOTA-IF7 was at least 15.6 TBq/mmol. The HPLC chromatogram of 68Ga-NOTA-IF7 was presented in Fig. 2. The resulting 68Ga-NOTA-IF7 was determined with a radiochemical purity of 95.4 %. The retention time of 68Ga-NOTA-IF7 in our gradient system was 17.7 min.
In vitro stability of the radiotracers
68Ga-NOTA-IF7 was stable in PBS at room temperature. After 2 h, the radiochemical purities were >95 % with HPLC analysis. We also tested the stability of 68Ga-NOTA-IF7 in mouse serum up to 2 h at 37 °C. After treating the serum samples with acetonitrile and centrifugation, the compound at 1, 30, 60 and 120 min constituted 92.1, 91.5, 90.3 and 89.3 % of radioactivity (Fig. 3). 68Ga-NOTA-IF7 was also stable in mouse serum up to 2 h at 37 °C. The result of the cell-binding assay indicated that with different concentrations of NOTA-IF7 it can obviously inhibit the 68Ga-NOTA-IF7 binding to U118 tumor cells. The specific binding rate of 68Ga-NOTA-IF7 with U118 tumor cells was 12 %.
Biodistribution studies
To evaluate tissue distribution characteristics of 68Ga-NOTA-IF7, we performed a biodistribution experiment using ICR mice. The data shown as the percentage administered activity (injected dose) per gram of tissue (%ID/g) in Table 1. 68Ga-NOTA-IF7 showed low accumulation in brain, heart and lungs. The liver uptakes were 9.43 ± 2.03, 5.35 ± 1.79 and 3.94 ± 1.66 %ID/g at 15, 30 and 60 min post-injection time, respectively. While the kidney uptakes were 9.07 ± 2.42 and 3.68 ± 1.01 %ID/g at 15 and 30 min post-injection. The radioactivity in blood, heart, liver decreased rapidly in the first 30 min. At 60 min after injection, the radioactivity concentration in blood was only 2.13 ± 1.26 %ID/g. The results of biodistribution indicate that 68Ga-NOTA-IF7 can be cleared rapidly (Fig. 4).
MicroPET imaging
After radiolabeling, we applied the imaging probe to in vivo PET imaging with U118 tumor bearing mice. The representative decay-corrected sagittal images at different time points after injection of the radiotracer are shown in Fig. 5. The U118 tumor was clearly visualized with good tumor-to-background contrast with the tracer as early as 30 min post injection (p.i.). The quantitative tracer uptake of tumor and muscle is summarized in Fig. 6, expressed as %ID/g ± SD.
Discussion
The ongoing interest in radiolabeled peptides and proteins as specific molecular probes mainly stems from the elevated numbers of high affinity receptors for peptides and proteins as found in many neoplastic and inflammatory tissues. The use of radiolabeled peptides and proteins for receptor imaging and tumor targeting in vivo has become an established method in diagnostic nuclear medicine [6].
Herein we describe the optimization of the 68Ga labeling of NOTA-IF7. The optimal labeling conditions were as followed: the pH of reaction solution was 4; the temperature of the reaction was 100 °C and the reaction time was 10 min. The labeled yield and radiochemical purity of 68Ga-NOTA-IF7 were satisfactory under the above conditions.
Radiochemical stabilities of radiopharmaceuticals are critical to their safe, effective use in the diagnosis and therapy of disease. 68Ga-NOTA-IF7 was stable in PBS at room temperature for 2 h. And only minor changes were observed in mouse serum up to 2 h at 37 °C. This rate of change was acceptable for clinical 68Ga labeled preparations and suitable for in vivo use at least 2 h.
The mouse U118 tumor model was selected for evaluating the characters of 68Ga-NOTA-IF7 since this tumor has good characteristics that make it a suitable experimental animal model for tumor MicroPET imaging. Tumor cells are easily transplanted into the mice. In vitro tests also revealed the ability of 68Ga-NOTA-IF7 to binding with the U118 cells. Biodistribution experiment showed that 68Ga-NOTA-IF7 could be eliminated from the body by the alimentary system.
To further confirm and evaluate the tumor accumulation of the tracer, the MicroPET imaging was performed in mice bearing U118 tumor. The ratios of the tumor to muscle uptake were 20.61 ± 0.31, 13.14 ± 0.21, 2.39 ± 0.12 at 15, 30 and 60 min post-injection, respectively. The U118 tumor was clearly visualized with good tumor-to-background contrast with the tracer during 30 min post injection. The above results indicated that 68Ga-NOTA-IF7 might be suitable for imaging of cancer with high tumor uptake, low nontarget uptake.
Conclusion
In conclusion, we have demonstrated the feasibility of using PET imaging probe to monitor tumor. 68Ga-NOTA-IF7 can be prepared without HPLC purification. The results showed that 68Ga-NOTA-IF7 is promising agent for PET imaging of U118 tumor. This preclinical study should be helpful in accelerating anticancer drug development and promoting the clinical translation of molecular imaging.
References
Lang LX, Li WH, Guo N, Ma Y, Zhu L, Kiesewetter DO, Shen BZ, Niu G, Chen XY (2011) Bioconjug Chem 22:2415–2422
Breeman WA, Verbruggen AM (2007) Eur J Nucl Med Mol Imaging 34:978–981
Jeong JM, Hong MK, Chang YS, Kim YJ, Cheon GJ, Lee DS, Chung JK, Lee MC (2008) J Nucl Med 49:830–836
Hatakeyama S, Sugihara K, Shibata TK, Nakayama J, Akama TO, Tamura N, Wong SM, Bobkov AA, Takano Y, Ohyama C, Fukuda M, Fukuda MN (2011) Proc Natl Acad Sci USA 108:19587–19592
Fedorova OS, Kuznetsova OF, Shatik SV, Stepanova MA, Belokon YN, Maleev VI, Krasikova RN (2009) Bioorg Khim 35:334–343
Wuest F, Kohler L, Berndt M, Pietzsch J (2009) Amino Acids 36:283–295
Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, Testa JE, Schnitzer JE (2004) Nature 429:629–635
Hatakeyama S, Sugihara K, Nakayama J, Akama TO, Wong SM, Kawashima H, Zhang J, Smith DF, Ohyama C, Fukuda M, Fukuda MN (2009) Proc Natl Acad Sci USA 106:3095–3100
De Sa A, Matias AA, Prata MI, Geraldes CF, Ferreira PM, Andre JP (2010) Bioorg Med Chem Lett 20:7345–7348
Tolmachev V, Altai M, Sandstrom M, Perols A, Karlstrom AE, Boschetti F, Orlova A (2011) Bioconjugate Chem 22:894–902
Cai GM, Jiang MJ, Zhang B, Zhou YY, Zhang LF, Lei JY, Gu XB, Cao GX, Jin J, Zhang RJ (2009) Biol Pharm Bull 32:440–443
Acknowledgments
This project was supported by the Science Foundation of Health Department of Jiangsu Province (H201226) and the Natural Science Foundation of Jiangsu Province, China (BK2010154).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gu, X., Cai, G., Zhang, R. et al. Synthesis and preliminary evaluation of 68Ga-NOTA-IF7 as a tumor imaging agent. J Radioanal Nucl Chem 303, 777–782 (2015). https://doi.org/10.1007/s10967-014-3459-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3459-5