Abstract
This work reports the synthesis, radiolabeling and biological studies of 99mTc(HYNIC–MFL)(tricine)(TPPTS) in tumor-bearing mice. The novel melphalan derivative was successfully synthesized by conjugation of HYNIC to melphalan. The ligand could be labeled by 99mTc in high yield to get 99mTc(HYNIC–MFL)(tricine)(TPPTS), which was very hydrophilic and was stable at room temperature. Biodistribution studies in tumor-bearing mice showed that 99mTc(HYNIC–MFL)(tricine)(TPPTS) accumulated in the tumor with favorable uptake and retention. The good accumulation in tumor tissue, high tumor/muscle ratios and satisfactory scintigraphic images highlight the potential of 99mTc(HYNIC–MFL)(tricine)(TPPTS) for tumor imaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The nitrogen mustards are an important class of DNA cross-linking agents, which are utilized in the treatment of many types of cancer [1]. The cytotoxicity of nitrogen mustards is due to their ability to alkylate DNA, RNA and proteins leading to the formation of DNA interstrand cross links [2, 3]. The prototype nitrogen mustard drug is mustine, which is no longer commonly in use. Other nitrogen mustards include cyclophosphamide, chlorambucil, uramustine, ifosfamide, melphalan and bendamustine.
Due to the tumor affinity of nitrogen mustards, they have been studied as potential tumor imaging agents after radionuclides labeling. D. Satpati et al. have reported a 99mTc(CO3)-labeled chlorambucil analog which shows good tumor uptake of 3.2 ± 0.3 % ID/g at 3 h p.i. However, the complex is not stable in vivo [4]. Our previous studies have confirmed the 99mTc(CO)3-labeled pegylated chlorambucil ([99mTc(CO)3(IDA-PEG3-CB)]−, IDA = iminodiacetic acid) shows good accumulation in tumor tissue with high tumor/muscle ratios. Its major disadvantage lies in the slow clearance from normal organs [5]. Another 99mTc(CO)3-labeled chlorambucil we have investigated is 99mTc(CO)3(His-CB). It exhibits high initial tumor uptake with certain retention, fast clearance from background, good tumor/background ratios and satisfactory scintigraphic images, all of which highlight the potential of 99mTc(CO)3(His-CB) as a tumor imaging agent [6]. Melphalan has been labeled with 125I directly to get [125I] Melphalan, which has a high affinity to be localized in the tumor site and meets most of the requirements necessary to be used as a successful diagnostic and therapeutic agent [7].
99mTc is the most widely used radionuclide for diagnostic imaging with SPECT because of its favorable physical properties (t 1/2 = 6 h, E γ = 140 keV), low cost, and widespread availability [8]. In search for a more suitable 99mTc-labeled tumor imaging probe based on the biomolecule melphalan, 6-hydrazinonicotinyl (HYNIC) was chosen as the chelating agent because it has been widely used in 99mTc labeled proteins and peptides on the basis of different coligands [9–12]. The 99mTc-HYNIC labeling is very effective using very small amount of ligand to form a stable result complex. For 99mTc-labeling, HYNIC was used as the bifunctional coupling agent whereas tricine and TPPTS (trisodium triphenylphosphine-3,3′,3″-trisulfonate) were used to stabilize the 99mTc-HYNIC core in 99mTc complex: 99mTc(HYNIC–MFL)(tricine)(TPPTS).
Experimental
Materials and methods
Melphalan was purchased from Sigma/Aldrich, China. Other reagents and solvents were from Beijing Chemical Reagents Company, China. All the chemicals were analytically pure and used without further purification. HYNIC–OSu (–OSu = –oxy succinimide) was synthesized according to the described method [13]. 1H NMR spectra were recorded with a 500 MHz spectrometer by using TMS (tetramethylsilane) as the internal standard. Mass spectrometry was performed on a Bruker Daltonics esquire 6000 mass spectrometer with electrospray-ionisation prob. A99Mo/99mTc generator was obtained from the China Institute of Atomic Energy. Thin layer chromatography (TLC) was performed on a polyamide strip developed by dichloromethane/methanol = 1/1 (V/V). High performance liquid chromatography (HPLC) analysis was carried out by using a Hitachi LC2000 System with Bioscan FC3200 radio-detector. The column (Kromasil 100-5C18, 250 × 4.6 mm) was eluted at a flow rate of 1.0 mL/min. Water with 0.1 % trifluoroacetic acid (A) and methonal with 0.1 % trifluoroacetic acid (B) mixtures were used as the mobile phase and the following gradient elution technique was adopted for the preparation (0 min 10 % B, 15 min 40 % B, 20 min 90 % B, 25 min 10 % B). The imaging studies were performed with Eplus-166 small animal SPECT/CT system (Institute of High Energy Physics, Chinese Academy of Sciences).
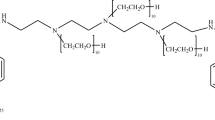
Synthesis of the ligand (Fig. 1)
The HYNIC–melphalan conjugate (HYNIC–MFL) was synthesized by reacting HYNIC–OSu with an equivalent amount of melphalan. The reaction is schematically shown in Fig. 1. To 10 mL of N,N-dimethyl formamidine (DMF) were added HYNIC–OSu (192 mg, 0.5 mmol) and melphalan (163 mg, 0.5 mmol), followed by addition of 100 μL of triethylamine (Et3N). The reaction mixture was stirred at room temperature over night. Then 10 mL of pure water was added. The precipitate was filtered, washed with DMF (0.1 mL) and ethanol (1 × 3 mL). The final product was collected and dried to give a yellow powder. The yield was 115 mg (40.2 %). 1H NMR (500 MHz, DMSO): δ = 2.95 (s, 6 H), 3.02 (m, 2 H), 3.67 (m, 9 H), 4.45 (bs, 1 H), 5.59 (d, 1 H), 6.65 (d, 2 H), 6.74 (d, 2 H), 7.15 (m, 2 H), 7.51 (d, 2 H), 7.96 (m, 2 H), 8.40 (bs, 1 H), 8.56 (s, 1 H), 10.99 (s, 1 H), ppm. ESI-MS: 571.38 (MH+), 593.71 (M + Na+).
Radiosynthesis of 99mTc(HYNIC–MFL)(tricine)(TPPTS) (Fig. 2)
To a solution of 0.1 mL of DMSO and 0.5 mL of PBS (pH 6) were added 1 mg of HYNIC–MFL, 25 mg of tricine and 5 mg TPPTS (tris(3-sulfonatophenyl)phosphine, sodium salt). Stannous chloride (10 μL, 3 mg/mL, pH 1) was added followed by addition of freshly eluted 99mTechnetium pertechnetate (111 MBq; 100 μL). The reaction mixture was heated at 100 °C for 15 min. After cooling to room temperature, the radiochemical purity (RCP) of the complex was checked by TLC and HPLC.
Determination of the partition coefficient
The partition coefficient was determined by mixing the complex with an equal volume of 1-octanol and phosphate buffer (25 mM, pH 7.4) in a centrifuge tube. The mixture was vigorously stirred for 10 min at room temperature, and was then transferred to an Eppendorf microcentrifuge tube. The tube was centrifuged at 8000 rpm for 10 min. Samples in triplets from n-octanol and aqueous layer were obtained, and were counted in a well γ-counter. The partition coefficients were calculated using the following equation: P = (activity in n-octanol)/(activity in aqueous layer). The final partition coefficient value was expressed as log P.
Stability studies
The room-temperature stability of the complex was determined by measuring the RCP at 25 °C at different time points (0, 1, 2, 4, 6 h) after preparation. Serum stability was determined by 99mTc(HYNIC–MFL)(tricine)(TPPTS) diluted 20-fold with freshly prepared murine serum, and the solutions were incubated at 37 °C for 6 h. At different time points (0, 1, 2, 4, 6 h), the serum was passed through a Sep-Pak C18 cartridge (waters), washed with 0.5 mL of water and eluted with 0.5 mL of acetonitrile containing 0.1 % trifluoroacetic acid. The combined aqueous and organic solutions were passed through a 0.22 μm Millipore filter and evaluated by radio-HPLC.
Biodistribution studies in tumor-bearing mice
Biodistribution characteristics of 99mTc(HYNIC–MFL)(tricine)(TPPTS) were evaluated using ICR mice bearing S180 cancer xenografts. In vivo growth was initiated by hypodermic injection of approximately 106 S180 cells into the left front leg of female ICR mice. Seven-eight days after inoculation, the tumor size was in the range of 0.5–0.8 g, and animals were used for biodistribution studies. A solution of the 99mTc(HYNIC–MFL)(tricine)(TPPTS) (100 μL, 185 KBq) was injected into the tumor-bearing mice (n = 20) via the tail vein. Five mice were sacrificed at 30, 60, 120 and 240 min post-injection (p.i.). The organs of interest and blood were collected, weighed and measured for radioactivity. The accumulated radioactivity in the tissue of organs was calculated in terms of percentage of injected dose per gram organ (%ID/g). The biodistribution data, tumor/muscle and tumor/blood ratios are reported as an average plus the standard variation. All biodistribution studies were carried out in compliance with the national laws related to the conduct of animal experimentation.
Imaging studies
The imaging studies of 99mTc(HYNIC–MFL)(tricine)(TPPTS) were performed using ICR mice bearing S180 cancer xenografts. A solution of the 99mTc(HYNIC–MFL)(tricine)(TPPTS) (100 μL, 14.8 MBq) was injected into the tumor-bearing mice via the tail vein. Multiple static scans were obtained at 30, 60, 120, 180, and 240 min p.i.
Results and discussion
Synthesis and radiosynthesis
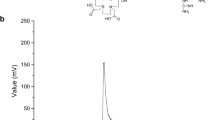
Derivatization of melphalan to the suitable precursor was essential for subsequent 99mTc-labeling. 99mTc-HYNIC is a very attractive 99mTc-labeling core for its much high labeling efficiency and very stable result complex [9–12]. In order to get the HYNIC–melphalan ligand (HYNIC–MFL), HYNIC was activated by n-hydroxysuccinimide (HOSu) and then reacted with melphalan. HYNIC–MFL was precipitated and purified by solvents washing. The 99mTc-labeling of HYNIC–MFL was very effectively through a simple method. When TLC was performed, 99mTc(HYNIC–MFL)(tricine)(TPPTS) moved to the solvent front (R f = 0.8–1.0), 99mTcO2·nH2O remained at the origin (R f = 0) and R f value for 99mTcO4 − was about 0.0–0.1. According to the TLC analysis, there was no observable 99mTcO2·nH2O impurity in the radiolabeling results. The HPLC pattern of 99mTc(HYNIC–MFL)(tricine)(TPPTS) is shown in Fig. 3. It was observed that the retention time was found to be 7.64 min. The mean radiochemical purity (RCP) of the complex was over 95 % after the preparation.
Partition coefficient (log P)
The partition coefficient (log P) of 99mTc(HYNIC–MFL)(tricine)(TPPTS) was obtained to be −2.70 ± 0.03, suggesting that it had very good hydrophilicity.
Stability of the complex
The RCP of the product was nearly constant (>95 %) in the reaction mixture at room temperature or in serum at 37 °C over the observed period of 6 h (Fig. 4), suggesting that the complex possessed a great stability in vitro.
Biodistribution studies
The data of biodistribution are summarized in Table 1. 99mTc(HYNIC–MFL)(tricine)(TPPTS) did exhibit tumor affinity with good accumulation and retention (30 min: 1.83 ± 0.36, 60 min: 1.76 ± 0.55, 120 min: 1.12 ± 0.16, 240 min: 0.98 ± 0.37 ID %/g). The complex was mainly excreted via hepatobiliary and especially renal route. The clearance from normal organs (except of kidneys and liver) was very fast, leading to low background at 4 h p.i. (blood: 0.36 ± 0.05, heart: 0.25 ± 0.01, spleen: 0.38 ± 0.14, lung: 0.62 ± 0.11, muscle: 0.15 ± 0.02 ID %/g). It had favorite tumor/muscle (T/M) ratios that increased up to 4 h p.i. (30 min: 2.69 ± 0.30, 60 min: 3.80 ± 1.00, 120 min: 4.77 ± 1.33, 240 min: 6.63 ± 2.02). Due to the fast blood clearance, the tumor/blood (T/B) ratios were also improved during the observation period (30 min: 0.59 ± 0.11, 60 min: 1.12 ± 0.28, 120 min: 1.42 ± 0.17, 240 min: 2.75 ± 1.08).
Imaging studies
In imaging studies, the tumor (indicated by arrows) was clearly visualized during the whole experiment with very high tumor-to-muscle contrast (Fig. 5), which was consistent with the results from ex vivo biodistribution studies.
Conclusion
In this report, we have synthesized a new imaging agent 99mTc(HYNIC–MFL)(tricine)(TPPTS) showing substantial promise for tumor scintigraphy, as significant accumulation is observed in S180 tumor bearing mice. The RCP of the complex was over 95 %. It had good hydrophilicity and was stable at room temperature. 99mTc(HYNIC–MFL)(tricine)(TPPTS) showed obvious tumor uptake and retention, good tumor/muscle ratios and satisfactory scintigraphic images, suggesting it would be a promising candidate for tumor imaging.
References
Panasci L, Xu ZY, Bello V, Aloyz R (2002) Anticancer Drugs 13:211–220
Osborne MR, Wilman DEV, Lawley PD (1995) Chem Res Toxicol 2:316–320
Hurley LH (2002) Nat Rev Cancer 2:188–200
Satpati D, Korde A, Venkatesh M, Banerjee S (2009) Appl Radiat Isot 67(9):1644–1649
Wang J, Yang J, Yan Z, Duan X, Tan C, Shen Y, Wu W (2011) J Radioanal Nucl Chem 287:465–469
Wang J, Yang J, Duan X, Zhang Y, Yang W, Liu Y (2012) Bioorg Med Chem Lett 22:7406–7409
Amin AM, Soliman SE, El-Aziz HA (2010) J Label Compd Radiopharm 53:1–5
Jurisson SS, Lydon JD (1999) Chem Rev 99:2205–2218
Liu S, Edwards DS, Barrett JA (1997) Bioconjug Chem 8:621–636
Abrams MJ, Juweid M, TenKate CI, Schwartz DA, Hauser MM, Gaul FE, Fuccello AJ, Rubin RH, Strauss HW, Fischman AJ (1990) J Nucl Med 31:2022–2028
Masahiro O, Yasushi A, Takahiro M, Yasushi F, Kazuma O, Tomoya U, Tsuneo S, Junji K, Hideo S (2001) Nucl Med Biol 28(3):215–224
Steffens MG, Oosterwijk E, Kranenborg MHGC, Manders JMB, Debruyne FMJ, Corstens FHM, Boerman OC (1999) J Nucl Med 40:829–836
Harris TD, Sworin M, Williams N, Rajopadhye M, Damphousse PR, Glowacka D, Poirier MJ, Yu K (1999) Bioconjug Chem 10(5):808–814
Acknowledgments
The work was financially supported by National Key Basic Research Program of China (2013CB932703) and National Natural Science Foundation of China (11575211, 21371172, 11475198, 21401198).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, J., Yang, W., Xue, J. et al. Synthesis and biological studies of 99mTc(HYNIC–MFL)(tricine)(TPPTS) as a novel tumor imaging agent. J Radioanal Nucl Chem 310, 1209–1213 (2016). https://doi.org/10.1007/s10967-016-4955-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-4955-6