Abstract
A novel anion-exchange chromatographic method for separation of radioiodine from an antimony target irradiated with 3He- or α-particles was developed, with separation yield of radioiodine amounting to 90 ± 5 % and its decontamination factor from the Te and Sb radionuclides to ~104. The optimized separation method developed was then applied to the production of 124I via the 121Sb(α,n)124I process using a highly enriched 121Sb target. Quality control tests showed that the separated 124I occurred >99 % as iodide and the longer lived impurities 126I and 125I amounted to 0.16 % and <0.05 %, respectively. The trace level of inactive Sb impurity was determined by ICP–OES.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The radionuclide 124I (T 1/2 = 4.18 days) is an important longer lived positron emitter. For its production several methods have been suggested [for a review cf. 1]. Whereas previously the 124Te(d,2n)124I process was commonly used, in recent years the emphasis has shifted over to the 124Te(p,n)124I reaction [2] which leads to the highest purity product achieved so far [cf. 3], though the yield is not high. A higher yield reaction, namely 125Te(p,2n)124I is associated with higher radionuclidic impurity [4]. The 3He- and α-particle induced reactions on isotopes of antimony also appear interesting [cf. 5–9]. In particular, the α-particle induced reactions on both 121Sb and 123Sb could possibly lead to 124I of acceptable purity, though the yields would be rather small. Investigations on the latter processes, however, have so far been limited only to cross section measurements.
This work deals with the medium-scale production of 124I starting from an antimony target. There were two distinct motivations behind this work.
-
(a)
To develop and optimize a method for chemical separation of radioiodine at tracer level from an antimony target using anion-exchange chromatography. The methods available for the separation of radioiodine from antimony were mostly based on dissolution of the target and isolation of radioiodine either by distillation or solvent extraction [cf. 10–17]. They were mainly applied to the production of 123I. With the use of the 124Te(p,2n)123I reaction in combination with the dry distillation of radioiodine from a 124TeO2 target [cf. 18] and, above all, with the advent of the 124Xe(p,x)123I process in 1980s for the production of 123I, other routes became less important [cf. 19] and consequently the wet chemical processing methods for radioiodine were almost forgotten. Recently the production of radioiodine via heavy-ion and α-particle irradiation of antimony was re-investigated but the separation method was again based on solvent extraction [20, 21]. El-Azony and Qaim [22], on the other hand, developed an anion-exchange method for the separation of 123I from a 123Te metal target irradiated with protons to produce 123I via the 123Te(p,n)-reaction [cf. 23, 24]. A somewhat similar method was used for the separation of 131I formed in the decay of 131Te [25]. As far as we know, the ion-exchange method has hitherto not been attempted for the separation of radioiodine produced in 3He- or α-particle bombardment of antimony.
-
(b)
To apply the developed and optimized separation method eventually to medium-scale production of 124I via the 121Sb(α,n)124I process. The optimum energy range for this process was recently assessed as E α = 21 → 14 MeV [cf. 5, 7, 8].
The investigations related to the two motivations are described below separately.
Development of a radioiodine separation scheme
Chemicals and detection equipment
The chemicals and reagents used in this work were as follows: Sb2O3 (Chempur, Karlsruhe; 99.99 %), anion-exchanger Amberlyst A21, Cl− form, 100–200 mesh (Dow Chemical), tetrabutyl ammonium bromide (TBAB) and hydrochloric acid, analytical grade (Fluka AG), sodium hydroxide (Merck; >99 %), ethyl acetate, methanol and ethanol (Sigma Aldrich; >99 %).
The equipment for measurement of radioactivity consisted of several PC-based high-resolution HPGe detectors (about 1.7 keV FWHM at 1,332 keV) coupled to multichannel analyzers. The γ-ray spectra were analyzed by the program GammaVision 5.1. (Both hard and software, by Ametek/Ortec, Oak Ridge, Tennessee, United States). For detection of macro quantities of Sb, an inductively coupled plasma–optical emission spectrometer (ICP–OES), ULTIMA2 ICP, Jobin–Yvon S.A., France, was used at the NRC, AEA, Cairo.
Radionuclide generation for tracer studies
For tracer studies on radioiodine separation, radionuclides were generated by cyclotron irradiations. About 300 mg of natSb2O3 was pressed under a pressure of 10 tons cm−2 to form a pellet of 13 mm diameter which was then placed in the cavity of a target holder, covered by a Cu foil, and tightened by a screw cap. For irradiation an extracted beam system at the Compact Cyclotron CV28 of the Forschungszentrum Jülich was used [26]. Thereby the target holder was located in the vacuum of the beam line; it was cooled at its back by a stream of water. Irradiation was done with 36 MeV 3He-particles at 1 µA for a duration of 1 h. The pellet was thick enough to absorb the whole energy of the 3He-particle beam.
After irradiation the pellet was dissolved in HCl (of different concentrations), adjusting the amount of antimony to 10 mg mL−1 of the solution. The important nuclear reaction products involved were [cf. 6]: 123Sb(3He,2n)124I, 123Sb(3He,3n)123I, 121Sb(3He,3n)121I \( \mathop \to \limits^{{EC,{\ss}^{ + } }} \) 121Te, 121Sb(3He,t)121Te and 121Sb(3He,α)120Sb. Out of all the radioactive products, the somewhat longer lived radionuclides 124I (T 1/2 = 4.18 days; Iβ += 22 %, EC = 78 %; E γ = 603 keV (60.1 %), 723 keV (40.1 %)), 121Te (T 1/2 = 16.78 days; EC = 100 %; E γ = 573 keV (80.3 %)) and 120Sb (T 1/2 = 5.76 days; EC = 100 %; E γ = 197 keV (88 %)) were chosen for tracer studies on separation procedures. Their decay data were taken from literature [27, 28]. The tracer nuclides 124I and 121Te were in no-carrier-added form. In contrast, the radionuclide 120Sb was in admixture with large amount of the natSb carrier. Furthermore, it should be noted that irradiation was done with 3He-particles because the α-particle irradiation did not produce 121Te in sufficient amount.
Development of chromatographic separation
Distribution studies were performed using the batch technique as well as column elution. Several anion-exchange resins like Amberlyst A21, Dowex 21k, Dowex 1x4 and Dowex 1x2 were tested but the emphasis was on Amberlyst A21. It is a weak base resin with dimethylamino groups and the counter ion OH−.
Determination of equilibration time
The effect of shaking time on the uptake of no-carrier-added 124I by the resin was investigated by using the batch technique. The aqueous phase under study was 5 mL of a solution prepared by dissolving the irradiated natSb2O3 in 7 M HCl. The solid phase was 50 mg of the resin. The batch contents were shaken for different times, a 1 mL sample from the aqueous phase was pipetted out and measured for 124I using HPGe detector high-resolution γ-ray spectrometry, as described in several earlier publications [cf. 2–6]. From the measured activity in the solution before and after shaking, the percentage uptake of 124I by the resin Amberlyst A21 as a function of time was determined. The result is shown in Fig. 1. It was concluded that a shaking time of about 60 min is sufficient to reach the equilibrium.
Determination of distribution coefficient
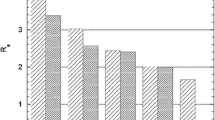
The distribution of the three elements under consideration, namely I, Te and Sb, between the solution and the resin was also determined using the batch technique. A 5 mL aliquot of the irradiated natSb2O3 solution in different concentrations of HCl was added to a fixed weight of the anion-exchanger (100 mg). The contents were shaken for 1 h to attain equilibrium, after which the two phases were separated. The activities of 124I and 121Te in solution prior to mixing and after attaining equilibrium were determined by γ-ray spectrometry. The difference in the activity was the fraction which was adsorbed on the sorbent. The distribution coefficient (K d) for each element was calculated using Eq. (1):
In contrast to 124I and 121Te, the activity of 120Sb was found to be very weak and consequently the results had a high uncertainty. The amount of antimony was therefore determined by inductively coupled plasma–optical emission spectrometry (ICP–OES). This was possible because macro quantities of antimony were involved.
The calculated distribution coefficients are shown in Fig. 2. At low HCl concentration (2 M) the distribution coefficient of 121Te was low, possibly due to the formation of the cationic species [Te(OH)3]+ [cf. 29]. At higher concentrations, tellurium(IV) begins to form anionic species of the type [TeOCl3]1− and [TeOCl4]2− [cf. 30] resulting in the increase of its K d value. In the case of antimony, the K d value increases with increasing HCl concentration, possibly due to the formation of the anionic species [SbCl6]1− and [SbCl6]3− [cf. 31]. For 124I, the K d value increases with HCl concentration, the species involved being iodide (I−).
From Fig. 2 it is obvious that at an HCl concentration of about 7 M, all the three elements under study are fairly strongly adsorbed on the resin Amberlyst A21.
Column elution studies
To a glass column (1 cm diameter, 30 cm length) filled with water to approximately half of its length, 3 g of Amberlyst A21 resin in a suspension of water was added. After the resin particles had settled down, the column was washed with double-distilled water, and equilibrated with 7 M HCl (the aqueous medium giving a relatively high K d value for each element under consideration). The irradiated target dissolved in 7 M HCl was then introduced in the column. The column was then flushed with 100 mL of 7 M HCl; i.e. about ten times the volume of the column. It was found that through this flushing about 97 % of antimony was eluted from the column. The amount of antimony in the eluted fraction was determined using ICP–OES analysis. It is interesting that the no-carrier-added 124I and 121Te were retained almost quantitatively on the column (as in batch experiments) but the natSb-carrier behaved differently. It showed good adsorption in batch experiment but was easily removed from the column, possibly via bulk effect or due to the reduced stability of the anionic complex. After the removal of Sb, 124I was eluted with different liquid phases and the amount of 124I removed was assessed via γ-ray spectrometry, as described above. The results are given in Table 1. A solution containing 5 mM tetrabutyl ammonium bromide (TBAB) in ethyl acetate (with a few drops of sodium dithionate) was found to be very efficient for the removal of 124I from the column. The phase transfer catalyst present in the elution medium might also be advantageous in given cases for labelling of some compounds with 124I. It was found that all 124I was eluted with 20 mL of the eluent. Thereafter 121Te was completely eluted with 40 mL of 2 M HCl. The elution profile is shown in Fig. 3. The fractions containing 124I were collected and by comparing the activity of 124I in the collected volume with that in the target solution, the yield of separation was determined to be 90 ± 5 %. The radionuclidic purity of 124I was ascertained by high-resolution γ-ray spectrometry: the decontamination factor from both 121Te and 120Sb was estimated to be ~104.
The method of radioiodine separation developed above thus consisted of dissolution of the irradiated Sb2O3 target in 7 M HCl, transfer to a column filled with the resin Amberlyst A21, elution of Sb with 100 mL of 7 M HCl, elution of radioiodine with 20 mL of 5 mM TBAB in ethylacetate, elution of radiotellurium with 30 mL of 2 M HCl, followed by collection of the radioiodine fraction for the desired application.
Production of 124I via the 121Sb(α,n)-process
In contrast to radionuclide generation for analytical studies, the medium-scale production of a cyclotron radionuclide for medical application demands development of high-current targetry, use of an efficient chemical separation method and stringent quality control of the separated radionuclide. The methodology developed for the production of 124I via the 121Sb(α,n)-process is described below.
High-current target development
About 300 mg of natSb2O3 was pressed at 10 tons cm−2 to obtain a pellet (as described above). The irradiation was, however, done in another target system at the Compact Cyclotron CV28 which was cooled in front by a He stream and at the back by flowing water [3]. For each irradiation a defocussed but collimated alpha-particle beam of primary energy 26.5 MeV was used which was fully stopped in the target. The beam current was varied from 1 up to 10 µA but the duration of each irradiation was kept constant at 1 h. At the end of each irradiation, the target was dismantled and the pellet was recovered. A visual inspection did not show any effect of radiation, i.e. the pellet was not damaged. The pellet was then dissolved in 10 mL of 7 M HCl. An accurately measured aliquot was taken and subjected to γ-ray spectrometry. The amount of 124I in the aliquot, and therefrom in the whole target solution, was then determined. Over the whole range of current used, the experimentally determined value of 124I, normalized for the chemical form and abundance of 121Sb in natSb2O3, was found to be within 10 % of the activity calculated from the excitation function of the 121Sb(α,n)124I-reaction [5, 7]. Thus, using the present target system irradiations with α-particle beams at a nominal current of 10 µA could be conveniently carried out. The target could hold even higher beam currents up to 20 µA, if a wobbled beam was used, though the experimental batch yield was then appreciably lower than the calculated integral yield.
Production under optimized conditions
In medium scale production experiments, enriched 121Sb (chemical purity >99.9 %, with major elemental impurities Mg, Si, S and Cu at a level of <0.01 %, supplied by Chemotrade GmbH, Düsseldorf) was used as target material. Its isotopic composition was: 121Sb = 99.45 %, 123Sb = 0.55 %. An exactly weighed amount of 200 mg of the material was pressed to obtain a pellet (as described above). It was placed in the target holder and covered by a Cu foil to degrade the energy of the incident 26.5 MeV α-particles to 22 MeV before entering the target material. Irradiation was then done with a defocussed but collimated beam for 10 h at a nominal beam current of 10 µA, using the well-cooled target system [3].
After the irradiation, the target was kept aside for a few days to let 123I decay which is the major radioactive impurity. Thereafter the irradiated pellet was dissolved in hot 10 mL of 7 M HCl with a few drops of H2O2 and the solution was transferred to a pretreated Amberlyst A21 column. Antimony was eluted with 100 mL of 7 M HCl. The fraction was collected for recovery of the enriched material. The radioiodine was then eluted with 20 mL of 5 mM TBAB in ethylacetate (with a few drops of sodium dithionate) and the fraction was collected. Finally the column was washed with 35 mL of 2 M HCl to remove radiotellurium. The long half-life of 124I allows concentrating the collected radioiodine fraction, if necessary for labelling purposes.
Yield and quality of 124I
The total amount of the radionuclide 124I present in the collected radioiodine fraction was determined by γ-ray spectrometry. The result for a typical production run is given in Table 2. The yield corresponds to about 75 % of the theoretically calculated value, though in absolute terms it is rather small.
The radionuclidic purity of 124I was also ascertained by γ-ray spectrometry. The impurity 123I (T ½ = 13.2 h) is the major concern which is formed via the 121Sb(α,2n)123I reaction. The impurity 125I (T ½ = 59.4 days) was not observed and thus only an upper limit was estimated. If at all, it could be formed only by the 121Sb(α,γ)125I reaction whose cross section is expected to be negligibly small [cf. 32]. The level of the impurity 126I (T ½ = 13.1 days) is very small. This radionuclide is formed through the 123Sb(α,n)126I reaction [cf. 9]. Since the content of 123Sb in the enriched sample was only 0.55 %, the low-level of 126I is understandable.
The radiochemical purity of the separated product was tested by two methods:
-
(i)
Thin layer chromatography (TLC). The technique described by El-Azony et al. [29] was used. The radioiodine solution was divided into two parts. To one part sodium iodide and to the other part sodium iodate (each about 30 mg) was added. Each mixture was spotted on a silica gel 60 plate (Merck) which acted as the stationary phase. A mixture of acetone/n-butanol/conc. NH3 (13.5 mol L−1)/H2O in the ratio of 65:20:10:5 was used as the mobile phase. The radioactivity (>99 %) was found exactly at the same spot as the sodium iodide carrier.
-
(ii)
High performance liquid chromatography (HPLC). Here the technique reported by Sheh et al. [33] was adopted. A LiChrosorb RP18 analytical column (250 mm × 4 mm Ø, Alltech) was used. The mobile phase consisted of a mixture of 90 % buffer (0.5 M potassium phosphate and 0.002 M tetrabutyl ammonium hydroxide, pH 7) and 10 % acetonitrile. The flow rate was 1 mL min−1. The retention time of radioiodine was compared with those of sodium iodide and sodium iodate. For mass detection, a UV 96 detector (Merck/Hitachi, L-4000) set at 254 nm was used and, for radioactivity measurement, γ-scintillation counting was employed. The results clarified that the radioactivity (>99 %) in the iodide form is separated at a retention time of 6 min while iodate is separated at 2 min. The obtained results were in good agreement with those obtained by TLC.
The chemical purity of the separated radioiodine was determined by ICP–OES analysis. Only Sb at a few micro gram level was detected. This level could possibly be further decreased by flushing the column with slightly more than 100 mL of 7 M HCl prior to the elution of radioiodine and radiotellurium from the column.
General discussion and conclusion
The above results show that the anion-exchange method of radioiodine separation from an antimony target reported in this work is simple and effective. It allows an easy recovery of the enriched target material and leads to radioiodine of high radiochemical and chemical purity. The radionuclidic purity of the product achieved via the 121Sb(α,n)124I reaction is comparable to that from the presently accepted reaction 124Te(p,n)124I. In both cases the 123I impurity is of some concern but it decays out much faster than 124I. The yield of 124I in the α-particle induced reaction is, however, by a factor of about six lower than that via the 124Te(p,n)124I reaction. A further disadvantage of the chromatographic separation method described here is the relatively large volume of the iodide solution (20 mL) as compared to the small volume (<1 mL) encountered in the dry distillation process [cf. 3]. This can be partly overcome by concentrating the eluted solution (see above), though with some loss of the activity. On the other hand, the cost of highly enriched 124Te target is about 10 times higher than that of the highly enriched 121Sb. Therefore, the 121Sb(α,n)124I reaction could possibly be used for local production if the rather expensive target material 124Te is not available.
References
Qaim SM (2010) Radiochemical determination of nuclear data for theory and applications. J Radioanal Nucl Chem 284:489–505
Scholten B, Kovács Z, Tárkányi F, Qaim SM (1995) Excitation functions of 124Te(p,xn)124, 123I reactions from 6 to 31 MeV with special reference to the production of 124I at a small cyclotron. Appl Radiat Isot 46:255–259
Qaim SM, Hohn A, Bastian Th, El-Azoney KM, Blessing G, Spellerberg S, Scholten B, Coenen HH (2003) Some optimization studies relevant to the production of high-purity 124I and 120gI at a small sized cyclotron. Appl Radiat Isot 58:69–78
Hohn A, Nortier FM, Scholten B, van der Walt TN, Coenen HH, Qaim SM (2001) Excitation functions of 125Te(p,xn) reactions from their respective thresholds up to 100 MeV with special reference to the production of 124I. Appl Radiat Isot 55:149–156
Hassan KF, Qaim SM, Saleh ZA, Coenen HH (2006) Alpha-particle induced reactions on natSb and 121Sb with particular reference to the production of the medically interesting radionuclide 124I. Appl Radiat Isot 64:101–109
Hassan KF, Qaim SM, Saleh ZA, Coenen HH (2006) 3He-particle induced reactions on natSb for production of 124I. Appl Radiat Isot 64:409–413
Tárkányi F, Takács S, Király B, Szelecsényi F, Andó L, Bergman J, Heselius SJ, Solin O, Hermanne A, Shubin YN, Ignatyuk AV (2009) Excitation functions of 3He- and alpha-particle induced nuclear reactions on natSb for production of medically relevant 123I and 124I radioisotopes. Appl Radiat Isot 67:1001–1006
Aslam MN, Sudár S, Hussain M, Malik AA, Qaim SM (2011) Evaluation of excitation functions of 3He- and alpha-particle induced reactions on antimony isotopes with special relevance to the production of iodine-124. Appl Radiat Isot 69:94–104
Uddin MS, Hermanne A, Sudár S, Aslam MN, Scholten B, Coenen HH, Qaim SM (2011) Excitation functions of α-particle induced reactions on enriched 123Sb and natSb for production of 124I. Appl Radiat Isot 69:699–704
Silvester DJ, Sugden J, Watson IA (1969) Preparation of iodine-123 by α–particle bombardment of natural antimony. Radiochem Radioanal Lett 2:17–20
Ragaini RC, Walters WB, Meyer RA (1969) Levels of 124Te from the decay of 4.2 d 124I. Phys Rev 187:1721–1732
Neirinckx RD (1970) The purification of cyclotron-produced 123I by liquid–liquid extraction. Radiochem Radioanal Lett 5:205–208
Dahl JR, Tilbury RS (1972) The use of a compact multi-particle cyclotron for the production of 52Fe, 67Ga, 111In and 123I for medical purposes. Int J Appl Radiat Isot 23:431–437
Hillman M, Nagy A, Weiss AJ (1973) Chemical effects of the decay of 121I to 121Te. Radiochim Acta 19:9–12
Vekic B, Horvath L, Horvat V, Vlatkovic M (1981) Production of I-123 by alpha-bombardment of natural antimony. Radiochem Radioanal Lett 47:35–44
Kozyreva-Alexandrova LS, Levin V, Malinin AB, Kurenkov VN, Zalessky VG, Shubyakova LP, Gusskov A (1982) A method of 123I production from an antimony target. Radiochem Radioanal Lett 51:215–222
Qaim SM, Stöcklin G (1983) Production of some medically important short-lived neutron-deficient radioisotopes of halogens. Radiochim Acta 34:25–40
Michael H, Rosezin H, Apelt H, Blessing G, Knieper J, Qaim SM (1981) Some technical improvements in the production of 123I via the 124Te(p,2n)123I reaction at a compact cyclotron. Int J Appl Radiat Isot 32:581–587
Qaim SM (1986) Recent developments in the production of 18F, 75,76,77Br and 123I. Int J Appl Radiat Isot 37:803–810
Maji S, Lahiri S (2007) Production of no-carrier-added 123I via heavy ion activation of natural antimony oxide. Radiochim Acta 95:133–136
Mandal S, Mandal A, Lahiri S (2012) Separation of nca 123,124,125,126I from alpha particle induced natural antimony trioxide target. J Radioanal Nucl Chem 292:579–584
El-Azony KM, Qaim SM (2008) Anion-exchange and solvent extraction studies on the separation of radioiodine with particular reference to the production of 123I via proton irradiation of 123Te metal target. J Radioanal Nucl Chem 275:275–284
Hupf HB, Eldridge JS, Beaver JE (1968) Production of iodine-123 for medical applications. Int J Appl Radiat Isot 19:345–346
Scholten B, Qaim SM, Stöcklin G (1989) Excitation functions of proton induced nuclear reactions on natural tellurium and enriched 123Te: production of 123I via the 123Te(p,n)123I process at a low energy cyclotron. Int J Appl Radiat Isot 40:127–132
Chattopadhyay S, Saha DS (2010) Recovery of 131I from alkaline solution of n-irradiated tellurium target using a tiny Dowex-1 column. Appl Radiat Isot 68:1967–1969
Blessing G, Weinreich R, Qaim SM, Stöcklin G (1982) Production of 75Br and 77Br via the 75As(3He,3n)75Br and 75As(α,2n)77Br reactions using Cu3As-alloy as a high-current target material. Int J Appl Radiat Isot 33:333–339
Browne E, Firestone RB, Shirley VS (1986) Table of radioactive isotopes. Wiley, New York
Qaim SM, Bisinger T, Hilgers K, Nayak D, Coenen HH (2007) Positron emission intensities in the decay of 64Cu, 76Br and 124I. Radiochim Acta 95:67–73
El-Azony KM, Mohty AA, Salah M (2004) Separation and purification of 131I from tellurium material using ion-exchange for preparing tetra-butyl ammonium iodide (131I). Appl Radiat Isot 61:1185–1188
Cotton FA, Wilkinson G (1972) Advanced inorganic chemistry, 3rd edn. Wiley, New York
Greenwood NN, Earnshaw A (1984) The chemistry of the elements. Pergamon Press, Oxford
Carlson RV, Daly PJ (1967) Excitation functions for (3He,γ) and (4He,γ) reactions. Nucl Phys A 102:161–176
Sheh Y, Koziorowski J, Balatoni J, Lom C, Dahl JR, Finn RD (2000) Low energy cyclotron production and chemical separation of “no carrier added” iodine–124 from a reusable, enriched tellurium-124 dioxide/aluminum oxide solid solution target. Radiochim Acta 88:169–174
Acknowledgments
This work was done under an Egyptian-German bilateral agreement and we are grateful to the concerned authorities in both countries for their support. A useful discussion with Prof. H. H. Coenen is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hassan, K.F., Spellerberg, S., Scholten, B. et al. Development of an ion-exchange method for separation of radioiodine from tellurium and antimony and its application to the production of 124I via the 121Sb(α,n)-process. J Radioanal Nucl Chem 302, 689–694 (2014). https://doi.org/10.1007/s10967-014-3270-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3270-3