Abstract
Activity concentrations of 238U, 232Th, 226Ra, 40K and 137Cs were measured in milk, egg, fruit and fish samples collected around a proposed site for setting up nuclear facilities, near Vishakhapatanam. The activity concentrations of the radionuclides ranged from 0.002 to 10.6, 0.002 to 2.8, 0.1 to 7.2, 3 to 110.8, 0.03 to 3 mBq g−1 for 238U, 232Th, 226Ra, 40K and 137Cs considering analysed food matrices. Natural uranium was measured in drinking water samples and the values were below 15 ppb. The average ingestion dose was 2.07 ± 2.01, 2.81 ± 4.38, 7.66 ± 8.24, 1.28 ± 0.84 and 0.04 ± 0.05 μSv year−1 for 238U, 232Th, 226Ra, 40K and 137Cs in milk, egg, fruit, fish and water. The ingestion dose received was the highest for milk, due to its high ingestion rate. It was observed that 226Ra is the largest contributor of measured radionuclides in this study for the different food matrices analysed due to its high dose conversion factor. The study was carried out as a part of baseline data generation for this region with which future changes in the radiological scenario can be compared.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural radiation and radioactivity is a fact of life. Naturally occurring radioactive materials are present in all compartments of the environment. Cosmic, terrestrial rays, inhalation and ingestion of radionuclides through air, water and food materials are the different sources of natural radiation [1]. Radioactivity enters the human body mainly by inhalation of radon and thoron and their decay products [2] and also by ingestion of primordial radionuclides and their progeny, like, 40K, 238U series and 232Th series radionuclides [3, 4]. Soils have different concentrations of radionuclides depending on their formation from the parent rocks, geographical location and by geochemical processes [5]. The radionuclides that are present in soils and fertilizers find their way to the human body via the food chain by atmospheric dispersion, gravitational settling, plant uptake and various other geochemical processes.
Foodstuffs are known to contain natural and artificial radionuclides that, after ingestion, contribute to an effective internal dose. The average natural radiation exposure to global population is 2.4 mSv year−1 [6]; whereas for Indian population it is reported as 2.3 mSv year−1 [7]. It has been estimated that nearly one-eighth of the mean annual effective dose due to natural sources is caused by the intake of food [6]. Hence it is extremely important to monitor the levels of radioactivity in foodstuffs to assess the ingestion dose to members of the public.
The present study was carried out as a part of the baseline survey around a proposed site for setting up nuclear facilities, near Vishakhapatanam (also known as Vizag). It is a prerequisite to establish the baseline radiological data at any nuclear facility before its commencement. The study area is situated around 17°31.417′N and 83°02.143′E in the South Eastern part of India, in the state of Andhra Pradesh. Granitic rocks that serve as good geochemical hosts for U, Th and K are predominant in Vizag. A major portion of this region is occupied by Proterozoic rocks covering a vast time span from 3,800 to 570 million years. The crust in this region shows an enrichment of rare earths with which U and Th are invariably associated in the form of minerals like monazite, samarskite, fergusonite and allanite. Sandstones, limestones, quartzites, slates with poor amounts of U, Th and K are also present [8].
This paper discusses the measurement of radionuclides in common foodstuffs and surface and ground water in the study area with high resolution gamma spectrometry (HRGS) system and laser fluorimetry and calculation of the consequent ingestion dose. Studies on natural radioactivity in food matrices have been reported from certain regions in India [9–12], but there is little data for this part of the country. Hence, this study will serve as the baseline for this area, with which future measurements can be compared to check any possible changes in the radiological scenario of this region.
Sampling and analytical techniques
Sample collection and processing
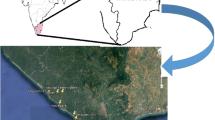
Foodstuffs were selected according to the consumption practices of local population residing around the study area. Samples were collected from local markets at different locations in the study area as shown in Fig. 1. Foodstuffs such as milk, fruits, eggs and fish were collected and processed according to the IAEA recommendations [13]. Food samples were washed with ultrapure water to remove soil and dust particles. Sample fresh weights were taken and they were put in a furnace for 24 h at 300 °C for ashing (preconcentration). Ash samples were powdered, homogenised and then transferred to plastic containers of standard geometry (polyethylene bottles of 6.5 cm diameter and 7.5 cm height). Water samples collected from the study area included surface water and ground waters. 5 L of water samples were each evaporated up to 250 mL and transferred to the standard containers. Food samples were sealed in standard geometry for 1 month, after drying and homogenization, to ascertain secular equilibrium between 226Ra and 224Ra and their respective daughter products. Samples were then counted on a gamma spectrometric system for calculation of radionuclide concentrations. Water samples were analysed by Laser Fluorimetry technique for estimation of total uranium concentration.
Radioactivity measurement
An n-type coaxial high-purity vertical germanium detector, of 100 % relative efficiency (DSG, Germany) was used for all the measurements. The detector was surrounded by 7.5 cm thick lead shield with inside dimensions 36 cm (l), 35 cm (b) and 36 cm (h). The resolution of the HRGS was 2.5 keV at 1,332 keV gamma energy of 60Co. IAEA Certified Reference Materials, RGU-I (235U and 238U and their daughter products) and RGTh-I (232Th and daughter products), were used for the energy and efficiency calibration of the HRGS [14]. The gamma spectra were acquired for 1,00,000 s and subjected to pulse height analysis by 8 K PC based MCA. Other details of the counting system and associated electronics are given elsewhere [15]. The energy calibrated detector was used for counting the standards (RGU-I and RGTh-I) with known activities. This enabled the calculation of detector efficiencies at particular energies, which were then subjected to curve fitting procedures to get efficiency versus energy plot. Identical geometry of the samples and standards were ensured by filling containers to the same volumes. The density correction factors were then applied to correct the difference in the densities of samples and standards. IAEA-330 (spinach) and IAEA-445 (spiked water) were analysed for quality control purposes. The obtained results were all within 95 % confidence interval of the recommended values.
The different chemical behavior of uranium and its daughters is responsible for their dissimilar transport behavior in the environment and differential plant uptake. Hence it is unlikely that uranium and its daughter radium will be in equilibrium in environmental matrices; eliminating the possibility of measurement of uranium from the gamma lines of 226Ra daughters. Since 238U emits a very weak low energy γ photon (49.56 keV, 0.064 %) it is measured by the γ rays of its immediate daughter product 234Th at 63.29 and 92.59 keV [16]. The 186.2 keV peak of 226Ra (γ 3.59 %) was used for the estimation of 226Ra after correcting for the contribution from 235U.
The 208Tl and 228Ac radionuclides were measured by the γ energies as given in Table 1 for the estimation of 232Th. The background spectra recorded were used to correct the net γ-ray photopeak areas for the isotopes of interest.
Activity concentration of each radionuclide was calculated using the following equation:
where C is the background subtracted net counts of the sample, E is the efficiency of the detector for the specific gamma ray energy, γ is the absolute transition probability of that specific gamma decay, M is the mass of sample in kg and T is the counting time in seconds.
Laser fluorimetry
Laser fluorimeter is a compact analytical instrument based on the principle of measuring the fluorescence of uranyl complex enhanced by addition of sodium pyrophosphate as fluorescence enhancement reagent. A sealed-off nitrogen laser is the excitation source which emits a very intense, short lived pulse (7 ns) of ultra violet light with wavelength 337.1 ± 0.1 nm. The maximum energy is 20 μJ at a repetition rate of 10 pulses s−1. This excites the uranyl complex in the aqueous medium. A PMT measures the green lights from excited uranium complex at 496–565 nm (4 peaks). The organic matter present in natural water also fluoresces when excited by the nitrogen laser, but has very short life time (<100 ns). The fluorescence of uranyl complex has a longer life time (>25 μs). By measuring the delayed fluorescence signal (a few microsecond after the laser pulse), the unwanted fluorescence of organic compounds are ignored and only the fluorescence of uranyl complex is collected by the time gated PMT. In addition to this, the fluorescence of organic matters has a wavelength maximum around 400 nm whereas that of uranyl complex is around 500 nm. Therefore, the wavelength filters at 450 nm significantly curtail the interferences of organic matters. The water samples were analyzed by standard addition method, in order to avoid the matrix effect. Sodium pyrophosphate (Na4P2O7·10H2O) was used as fluorescence enhancement reagent, as well as a complexing agent. The details analysis protocol are given elsewhere [17].
Estimation of committed effective dose (CED)
Radiation dose due to intake of radionuclides through ingestion pathway was calculated using IAEA dose conversion factors for adults [18]. The daily dietary intake was estimated by using prescribed food intake rates as given by IAEA [19]. The daily intake values considered were 100 mL milk, 20 g fruits, 6 g eggs and 12 g fish. The daily water intake was considered as 4.05 L [19]. The annual radiation ingestion dose was calculated by using the following formula [20].
where DCF is the dose conversion factor to convert activity to dose for corresponding intake.
Results and discussions
Activity concentrations
The activity concentrations of 238U, 232Th, 226Ra, 40K and 137Cs were 2.58 ± 2.26, 1.11 ± 1.19, 1.78 ± 0.67, 8.78 ± 11.55 and 0.24 ± 0.44 mBq g−1 in milk, 2.84 ± 3.37, 0.38 ± 0.22, 1.94 ± 1.20, 24.46 ± 8.54 and 0.93 ± 1.23 mBq g−1 in eggs, 5.30 ± 3.63, 0.80 ± 0.77, 5.06 ± 1.35, 43.78 ± 55.99 and 0.35 ± 0.31 mBq g−1 in fruits and 0.22 ± 0.38, 0.36 ± 0.6, 0.79 ± 0.59, 29.63 ± 14.52 and <0.03 mBq g−1 in fish, respectively. The data are represented by Box-whisker plot in Fig. 2. It can be observed from the figure that 232Th and 137Cs values lie within a narrow range compared to those of 238U and 226Ra. The activity concentrations of 40K lie within a wider range. The wide range of data values in the foodstuffs can be attributed to the variety of food stuff, the area/location of origin of the foodstuff and the transfer of a radionuclide to a particular food item. The lowest concentrations of radionuclides (except 40K) were observed for fish compared to other food items, as can be noted from Table 2. Highest concentrations were observed for 40K and lowest for 137Cs in the food materials. The U concentrations measured in drinking water samples were all below the guideline value of 15 ppb set by WHO [21] and 60 ppb set by AERB [22]. 79 % of the drinking water samples had 238U activity concentrations below 50 mBq L−1 as shown in Fig. 3. This constituted eleven out of fourteen samples. These values are comparable with other reported values worldwide except a few high vales [6].
The data obtained from the present study are compared to activity concentrations of radionuclides obtained from studies carried our worldwide in Table 3. The values are comparable with that reported for India in UNSCEAR [6]. The activity concentrations of radionuclides are in the range of the global values but lesser than the reported data in a high background radiation area in Nigeria [23, 24]. The values in this region are comparable with those reported in Punjab and Bombay in India but lesser than those from a HBRA in Odisha [12]. This can be attributed to the geochemistry of the region and the food items considered [25]. The baseline values observed at this site can be compared with other baseline studies carried out at Tamil Nadu [37] and Tarapur [38] in India and Lebanon [36]. In Tamil Nadu and Tarapur the studies have been carried out before the commencement of nuclear power plants, similar to the present study. Rao et al. [39] have reported data from Tarapur after 25 years of commencement of the facility, comparing their data with pre-operational values. This shows the significance of baseline studies and their utility in the years to come, after the commencement of a facility.
Committed effective dose
The average CEDs for the different radionuclides have been tabulated in Table 4. The highest CEDs were obtained for milk. This is due to the higher ingestion rate for milk (100 g day−1) compared to the other food matrices analysed, as given by IAEA [19]. The average ingestion dose received by 238U and 232Th in milk, eggs, fruits and fish make up for a total CED of 17.53 μSv year−1, which is higher than the reported annual effective dose of 0.54 μSv year−1 due to ingestion by the Asian population [26]. The CEDs due to 238U, 232Th, 226Ra, 40K and 137Cs were 10.37, 11.24, 30.65, 5.10 and 0.18 μSv/y considering milk, eggs, fruits, fish and water intake. These values are comparable with the CED values reported for Jaduguda [11] and Tamil Nadu [36] region. A total CED of 53.46 μSv year−1 was obtained considering all radionuclides in milk, eggs, fruit and fish. This is lower than that of 180.08 μSv year−1 in Pakistan [27], 109.83 μSv year−1 in Korea [28], 186 μSv year−1 in Lebanon [29], 362 μSv year−1 in Tenerife, Spain [30], 360 μSv year−1 in Tanzania [31], 476 μSv/y in Jos Plateau, Nigeria [23], 700 μSv year−1 in Cameroon [32]. This is because of low intake of the food matrices considered in this study. The large variation in CEDs is mainly due to the geochemical and crustal characteristics of the environment and the dietary and lifestyle choices of the local population.
Contribution of the individual radionuclides to the CEDs in different food matrices are represented in Fig. 4. The 238U, 232Th, 226Ra, 40K and 137Cs contributions ranged from 2.03 ± 3.42 to 13.86 ± 16.44, 8.7 ± 8.35 to 27.62 ± 29.56, 44.62 ± 33.18 to 66.99 ± 17.9, 5.87 ± 7.73 to 36.91 ± 18.09 and 0.07 ± 0.001 to 1.31 ± 1.73 %, respectively. The high degree of associated uncertainty is due to the high variation of radionuclide activity concentrations in a given matrix considering the number of samples of that matrix analysed during this study. This high degree of variation in radionuclide activity concentration is typical for environmental samples and may occur due to preferential uptake and accumulative properties of a radionuclide in a matrix which again depends on the soil characteristics of the region. It can be observed that the highest dose is obtained from 226Ra in all food matrices. Although the concentration of 226Ra is smaller in the matrices than 40K, its contribution is higher due to the higher value of DCF for adults (2.8 × 10−7 Sv Bq−1) [18] as compared to the other radionuclides.
Contribution of the radionuclides to the average CED combining all the food matrices analysed is shown in Fig. 5. It can be observed from the figures that that 226Ra is the major contributor, making up 56 and 60.3 % for the non-vegetarian and vegetarian diets of the dose considering the food matrices analysed. This is due to its higher DCF value. The smallest contribution is due to 137Cs. This is due to its lower concentration in the food matrices, apart from its small DCF value (1.3 × 10−8 Sv Bq−1) [18]. It can also be observed that the contribution of 226Ra, 238U and 232Th increase from non-vegetarian to vegetarian food, whereas contribution of 40K and 137Cs reduces. This is because of the variation of their corresponding concentrations in the food matrices and their variable intake rates.
Conclusion
Activity concentrations of 238U, 232Th, 226Ra, 40K and 137Cs were measured in various food matrices and the corresponding ingestion dose was evaluated. The activity concentrations of the radionuclides ranged from 0.002 to 10.6, 0.002 to 2.8, 0.1 to 7.2, 3 to 110.8, 0.03 to 3 mBq g−1 for 238U, 232Th, 226Ra, 40K and 137Cs considering milk, eggs, fruit and fish. The U concentrations measured in drinking water samples were all below the guideline value of 15 ppb set by WHO. The activity concentrations were comparable with reported values in studies carried out globally. Highest ingestion doses were obtained from milk, due to its higher intake rate. The total CED of 53.46 μSv year−1 was obtained for milk, fruit, fish and eggs. The highest contributor to the average ingestion dose was 226Ra for the different food matrices analysed due to its high value of dose conversion factor. The contribution of 226Ra, 238U and 232Th increase from non-vegetarian to vegetarian food, whereas contribution of 40K and 137Cs reduces.
References
Benville A, Lowder AM (1987) Human population exposures to cosmic radiation. In: 4th International conference on the natural radiation environment, Lisbon, Portugal
Misdaq MA, Ezzahery H, Elabboubi D (2001) Determination of equivalent dose rates and committed effective doses in the respiratory system from the inhalation of radon decay products by using SSNTD and a dosimetric compartmental model. Radiat Prot Dosim 93:347–355
Fisenne IM, Perry Decker KM, Keller HW (1987) The daily intake of 234,235,238U, 228,230,232Th and 226,228Ra by NewYork City residents. Health Phys 53:357–363
Shiraishi K, Tagami K, Muramatsu Y, Yamamoto M (2000) Contributions of 18 food categories to intakes of 232Th and 238U in Japan. Health Phys 78:28–36
Tzortzis M, Tsertos H (2004) Determination of thorium, uranium and potassium elemental concentrations in surface soils in Cyprus. J Environ Radioact 77:325–338
UNSCEAR (2000) Sources and effects of ionizing radiation, Report to General Assembly. United Nations, New York
Puranik VD (2005) Natural radiation and radioactivity In: Proceeding of 15th national symposium of environment, pp 42–48
Vinod Kumar A, Sahoo SK, Sumesh CG, Krishna NS, Tripathi RM, Puranik VD, Kushwaha HS (2010) Assessment of environmental gamma radiation and radon levels in and around the new BARC campus, Visakhapatnam. BARC Report BARC/2010/I/003
Ramachandran TV, Mishra UC (1989) Measurement of natural radioactivity levels in Indian foodstuffs by gamma spectrometry. Int J Rad Appl Instrum A 40(8):723–726
Kumar M, Prasher S, Singh S (2009) Uranium analysis in some food samples collected from Bathinda area of Punjab, India. Indian J Phys 83(7):1045–1050
Tripathi RM, Sahoo SK, Jha VN, Kumar R, Shukla AK, Puranik VD, Kushwaha HS (2010) Radiation dose to members of public residing around uranium mining complex, Jaduguda, Jharkhand, India. Radiat Prot Dosim. doi:10.1093/rpd/ncq496
Lenka P, Sahoo SK, Mohapatra S, Patra AC, Dubey JS, Vidyasagar D, Tripathi RM, Puranik VD (2012) Ingestion dose from 238U, 232Th, 226Ra, 40K and 137Cs in cereals, pulses and drinking water to adult population in a high background radiation area. Radiat Prot Dosim, Odisha, India. doi:10.1093/rpd/ncs115
IAEA (1989) Measurement of radionuclides in food and the environment, a guide book. Technical Report Series 295, Vienna
Shukla VK, Chinnaesakki S, Shanbhag AA, Sartandel SJ, Srivastava GK, Khan AH, Puranik VD (2004) In: Proceedings of the national symposium on environment, pp 445–448
Chakrabarty A, Tripathi RM, Puranik VD (2009) Occurrences of NORMS and 137Cs in soils of the Singhbhum region of Eastern India and associated radiation hazard. Radioprotection 44(1):55–68
Kim KH, Burnett WC (1985) 226Ra in phosphate nodules from the Peru/Chile seafloor. Geochim Cosmochim Acta 49:1073–1081
Patra AC, Mohapatra S, Sahoo SK, Lenka P, Dubey JS, Tripathi RM, Puranik VD (2013) Age dependent dose and health risk due to intake of uranium in drinking waters from Jaduguda, India. Radiat Prot Dosim 1–7, doi:10.1093/rpd/ncs328
IAEA BSS (1996) International basic safety standards for protection against ionizing radiation and for safety of radiation sources. Safety Series no. 115
IAEA-TECDOC-1005 (1998) Compilation of anatomical, physiological and metabolic characteristics for a Reference Asian man, Volume 1: Data summary and conclusions
Bronzovic M, Marcovic G (2005) Age-dependent dose assessment of 226Ra from bottled water intake. Health Phys 88(5):480–485
AERB (2004) Directive for limit on uranium in drinking water. Atomic Energy Regulatory Board, India, India
WHO (2004) Guidelines for drinking water—Water quality, vol 1, 3rd edn, Geneva
Jibiri NN, Farai IP, Alausa SK (2007) Activity concentrations of 226Ra, 228Th and 40K in different food crops from a high background radiation area in Bitscichi, Jos Plateau. Nigeria Radiat Environ Biophys 46:53–59
Arogunjo AM, Ofuga EE, Afolabi MA (2005) Levels of natural radionuclides in some Nigerian cereals and tubers. J Environ Radioact 82:1–6
Sankaran AV, Jayaswal B, Nambi KSV, Sunta CM (1986) U, Th and K distributions inferred from regional geology and the terrestrial radiation profiles in India. BARC Report
Iyengar GV, Kawamura H, Dang HS, Parr RM, Wang JW, Akhter P, Cho SY, Natera E, Miah FK, Nguyen MS (2004) Estimation of internal radiation dose to the adult Asian population from the dietary intakes of two long-lived radionuclides. J Environ Radioact 77:221–232
Akhter P, Rahman K, Orfi SD, Ahmad N (2007) Radiological impact of dietary intakes of naturally occurring radionuclides on Pakistan adults. Food Chem Toxicol 45:272–277
Min-Seok Choi, Xiu-Jing Lin, Lee Sun Ah, Wan Kim, Hee-Dong Kang, Sih-Hong Doh, Do-Sung Kim, Dong-Myung Lee (2008) Daily intakes of naturally occurring radioisotopes in typical Korean foods. J Environ Radioact 99:1319–1323
Nasreddine L, El Samad O, Hwalla N, Baydourn R, Hamzé M, Parent-Massin D (2008) Activity concentrations and mean annual effective dose from gamma emitting radionuclides in the Lebanese diet. Radiat Prot Dosim 131(4):545–550
Hernández F, Hernández-Armas J, Catalán A, Fernández-Aldecoa JC, Landeras MI (2004) Activity concentrations and mean annual effective dose of foodstuffs on the island of Tenerife, Spain. Rad Prot Dosim 111(2):205–210
MIwilo NA, Mohammed NK, Spyrou NM (2007) Radioactivity levels in staple foodstuffs and dose estimates for most of the Tanzanian population. J Radiol Prot 27:471–480
Ele Abiama P, Ben-Bolie GH, Amechmachi N, Najib F, El Koukhi t, Owono Ateba P (2012) Annual intakes of 226Ra, 228Ra and 40K in staple foodstuffs from a high background radiation area in the southwest region of Cameroon. J Environ Radioact 110:59–63
IAEA TRS 295(1989). Measurement of Radionuclides in Food and the Environment. A Guidebook. IAEA Vienna
Bolca M, Saç MM, Çokuysal B, Karal T, Ekdal E (2007) Radioactivity in soils and various foodstuffs from the Gediz River Basin of Turkey. Radiat Meas 42:263–270
Hosseini T, Fathivand AA, Abbasisiar F, Karimi M, Barati H (2006) Assessment of annual effective dose from 238U and 226Ra due to consumption of foodstuffs by inhabitants of Tehran city, Iran. Radiat Prot Dosim 121(3):330–332
El Samad O, Alayan R, Baydoun R, Zaidan W (2012) Radiation baseline levels in Lebanon: environmental survey and public dose assessment. Leban Sci J 13(2):37–48
Ross EM, Raj YL, Wesley SG, Rajan MP (2013) Selected natural and fallout radionuclides in plant foods around the Kudankulam Nuclear Power Project, India. J Environ Radioact 115:201–206
Kamath PR, Bhat IS, Khan AA, Ganguly AK (1968) Pre-operational search for Baseline radioactivity, critical food and population group at the Tarapur Atomic Power station site. In: Proceedings of the 1st international congress of radiation protection, pp 1111–1125
Rao DD, Baburajan A, Sudheendran V, Verma PC, Hegde AG (2010) Evaluation and assessment of 25 years of environmental radioactivity monitoring data at Tarapur (India) nuclear site. J Environ Radioact 101:630–642
Acknowledgments
The authors gratefully acknowledge the guidance and encouragement of Dr. D. N. Sharma, Director, Health Safety and Environment Group, BARC. The help and support received from Prof. Someswara Rao N. and his team of Andhra University, Visakhapatnam and Shri C. G. Sumesh and Shri N. S. Krishna of HS&EG, BARC during sample collection and processing are acknowledged. Suggestions and help from other colleagues during the preparation of this manuscript are also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patra, A.C., Mohapatra, S., Sahoo, S.K. et al. Assessment of ingestion dose due to radioactivity in selected food matrices and water near Vizag, India. J Radioanal Nucl Chem 300, 903–910 (2014). https://doi.org/10.1007/s10967-014-3097-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3097-y