Abstract
The adsorption and kinetic mechanism of uranium (U) and thorium (Th) in seawater-sediments system of Mumbai Harbour Bay (MHB) has been studied by K d values of two sets of experimental determinations using a batch experiment. After equilibrium time (7 days), under static conditions, K d for U and Th ranged from 25,030 to 55,662 mL/g (mean: 42,140 mL/g) and 24,926 to 38,561 mL/g (mean: 34,256 L/kg), respectively. Extraction studies showed that both U and Th were strongly bound to sediment components due to insignificant difference in their mean concentration in equilibrium solution. Rate constants (k) for transfer between seawater and the exchangeable fraction were found to be similar for the sediments as 1.02 ± 0.03 day−1 for uptake of U and Th. The resulting adsorption data were fitted to Freundlich, linear and Langmuir isotherm models. All the three models showed a significant correlation (R 2 >0.9), indicating that there is more complex relationships with adsorption behavior of U and Th on sediment surface. Since, the Freundlich constant (n) for U and Th was found to be closer to unity. Therefore linear model was observed to be highly suitable. Based on the linear model, the distribution coefficient (k d) of Th was about 1.5 times higher than U. According to Freundlich model, sorption of U in sediments observed to be higher than Th. However, Langmuir model considered in opposite to Freundlich and showed a higher value of K L constant for Th than U. The pH (water) of the sediments ranged from 7.8 to 8.2 and the estimated total carbon (determined by C H N S–O elemental analyser) ranged from 1.7 to 3.4 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since 232Th and 238U have been present over the time of existence of present marine systems, their chemical speciation and sorption behavior can provide data for development of predictive models for the long-term behavior of the synthetic radionuclides [1]. The uranium (U) contents and its mobility in aqueous systems is mainly controlled by the pH, alkalinity, the oxidation reduction potential (ORP) and the type of complexing agents present, such as carbonates, phosphates, vanadates, fluorides, sulfates and silicates, etc. [3]. The main oxidation states of U in natural water systems are U(IV) and U(VI). Under reducing aquatic environment (anoxic waters), usually, U(IV) minerals are the most abundant in U ore (uraninite, pitchblende, coffinite, etc.), but lead to low concentrations due to formation of its low solubility aqueous complexes. In contrast, in oxidized waters (Eh >200 mV), dissolved U mainly occurs as uranyl ions (UO2 2+), which form highly soluble hydroxide and carbonate complexes of varying stoichiometry as a function of pH and the partial pressure of CO2(g). At low pH, adsorption of uranyl cation (UO2 2+) is generally negligible and increases with increasing pH usually in the pH range of 4–6 [4]. In pure carbonate free aquatic environment with a U concentration of 10−8 M, mononuclear species (UO2 +, UO2OH+ and UO2(OH)2 0 dominate at all pH values. But in case of high U(VI) concentrations, the polynuclear species like, (UO2)3(OH)5 +) become the major hydroxyl complexes. In most natural waters, the uranyl carbonate complexes replace the U(VI)-hydroxyl complexes above pH 6–7 with a partial pressure of CO2 of 10−3.5 bar (normal atmospheric pressure) and with a typical ground water CO2 pressure (10−2 bar). In uranyl-hydroxide-carbonate system, the UO2CO3 0, UO2(CO3)2 2− and UO2(CO3)3 4− complexes predominate in the pH range of 6–8 [2].
Thorium (Th) is found in the nature only as a tetravalent cation concentrating in natural sediments either in detrital reinstate minerals such as monazite, rutile and thorianite, or adsorbed onto natural colloidal sized materials [1]. Th ion is largely hydrolyzed at pH above 3.2 and the hydroxy complexes are involved in the sorption process. The adsorption of Th on clays, oxides and organic matter increases with increasing pH and is completed at pH 6.5. In neutral to acid waters, Th adsorption is less on clays than on solid humic acid [1]. Studies on the effect of temperature, bicarbonate concentration and U concentration on the sorption of U to a number of pure minerals were conducted where idealised distribution coefficients are calculated from Freundlich isotherms [3–5].
Adsorption is one of the most important chemical processes in sediments. Sorption also affects the electrostatic properties of suspended particles and colloids. The electrostatic properties affect coagulation and settling [6]. Sorption reactions on sediment mineral surfaces potentially attenuate toxic sediment solution [7]. Sorption isotherm techniques provide useful information about the soil/sediment retention capacity and the strength by which the sorbate is held onto the soil/sediment.
Materials and methods
Sampling sites and sample collection
Four bottom sediment samples were collected from four locations of Mumbai Harbour Bay (MHB) which is the ultimate recipient of sewerage and effluents discharged from the chemical industries and very low level of radioactive effluent from Effluent Treatment Plant (ETP) of Bhabha Atomic Research Centre (BARC). MHB [latitude (19.5°–20°N), longitude (72.5°−73°E)] is a triangular mass of brackish water which widens out and opens to the Arabian sea in the South and narrow at the Northern end, where it is fed partially by river Ulhas. Along the east and west sides of the bay, many industrial units like textile mills of South and Central Mumbai, the petrochemical, fertilizer and thermal plants at Chembur and the pharmaceutical and chemical industries at Airoli, Vikhroli, Bhandup and Mulund.
Sample preparation and analysis
The collected raw sediment samples of 1 kg were dried at 110 °C for 24 h, powdered, homogenized and sieved through 110 mesh sizes. The powdered samples were thoroughly mixed with each other and prepared for two sets. The powdered sediment materials were washed thrice with deionised water for 7 days. The solid phase was allowed to settle by gravity and the washing solution was discarded. After washing, sediment samples were further dried at 110 °C, placed in conical flasks and stored as stock samples for experimental work. The moisture content of sediment was also determined. pH of sediment in water (1:2.5) was determined using precalibrated standard field equipment (Model: AM-200 GPS Aquameter, UK make). Total carbon in the sediment samples were estimated using C H N S–O elemental analyser (Flash EA 1112 Series, Thermo Finnigan, Italy). About 0.25 g of powdered dried samples were placed in polytetrafluoroethylene (PTFE) digestion vessels with 5 mL of HNO3 (MERCK, 65 %), 3 mL of HF (MERCK, 48 %) and 2 mL of hydrogen peroxide (H2O2) in Teflon beakers. After that samples were completely destructed in a closed microwave digestion system (Milestone Srl, Model Ethos 1, Italy) under sediment digestion program setting accordingly to manual from manufacture. The resulting solution after centrifuged was transferred to polypropylene tubes and diluted up to 50 mL with Milli-Q water. The concentration of heavy metals (Cu, Fe, Mn, Ni and Co) and major elements (Na, K, Mg, Ca and Cs) was determined by Ion-chromatography system (DIONEX, 600) using UV–Visible detector and conductivity suppressor. Clay minerals and minerals present in sediments of MHB were identified using powder X-ray diffractrometer (APD 2000 PRO, Italy make).
Adsorption experiments
In the present study, the batch method was chosen since it offered more rapid data, through a simpler way [3]. This technique consists of shaking an amount of sediment with a solution containing a certain radionuclide concentration until equilibrium is attained. In this method, experiments were conducted to determine the distribution coefficient of U and Th in sediments. Desired strength of standard of U and Th were prepared using stock solutions (1 g/L) of UO2(NO3)2 and Th(NO3)4. For this study, 5 g dried sediment samples was placed in each of seven empty conical flasks and equilibrated for 7 days with 150 mL of sea water containing 10, 20, 30, 40, 50, 75 and 100 mg L−1 of U and Th contents followed by shaking using end-over end shaker (model: 300, Korea make) at 25 ± 1 °C. After equilibration time, the samples of each set were centrifuged, filtered through 0.45 μm filter paper and supernatant analysed for U and Th. The pH of the equilibrated solution ranged from 7.8 to 8.1. Seawater samples were also analyzed for major cations and anions. Seawater of MHB was used as contact solution for sorption studies. Table 1 shows the physico-chemical characteristics of seawater of MHB. Sample preparations, procedures and conditions for the experiments were the same to produce reproducible results in order to make comparisons. Prior to the experiments, Th and U concentrations in sediments as a background concentration were determined using gamma spectrometry system.
Measurement of U and Th in equilibrium solution
The concentration of U in aliquots of equilibrium solution was measured by a laser fluorimeter (Quantalase Laser Fluorimeter, Indore, India) in which a pulsed nitrogen laser is used to excite uranyl species at 337.1 nm. The instrument was calibrated in the range of 1–100 μg/L using a stock solution of UO2(NO3)2 standard. The blank sample containing the same amount of fluorescing reagent was also measured for U concentration. 5 % phosphoric acid in ultra pure water was used as fluorescence reagent. All the reagents used for experimental work were of ultrapure/analytical grade, by Merck, Mumbai, India. Quality assurance was made by analysis of IAEA standard reference materials, spike recovery, replicate analysis and cross method checking. Relative standard deviation (RSD) was calculated to be 8–12 %. To avoid the matrix interferences, analyses were carried out using standard addition method.
Th was determined by loading the aliquots of the equilibrium solution on anion exchange column (Dowex resin, 100–200 mesh, 1 cm dia × 8 cm long) preconditioned in 8 N HCL. The column was then eluted with 8 N HCL which contains Th, Ra and other divalent an effluents. This fraction was again allowed to reload with 8 N HNO3 to separate Ra and eluted with 8 N HCL. Th in eluents was estimated by precipitating with ferric hydroxide followed by centrifugation. The precipitate was washed, dried, weighed in stainless steel planchet and estimated by gross alpha counter. For assuring the purity of Th an aliquot of the separated sample was also electroplated and estimated by alpha spectrometry system.
Distribution coefficient (K d) and Rate constant (k)
The distribution of an element between two phases can be described by the distribution coefficient, K d (mL/g), defined as the ratio between the concentration of the element in the solid phase and the concentration in the liquid phase after equilibrium achievement. The distribution coefficient K d was calculated according to
Assuming radioactive decay of U and Th in seawater-sediment system follows first-order process. The rate constant for first order kinetic reaction can be explained by the following equation,
Amount of U and Th adsorbed by the sediments was calculated using the following equation,
where A is the adsorption of contaminants in sediment; C i is the initial concentration of contaminant in the solution; C eq is the final concentration in solution after reaching equilibrium, V is the volume of the contact solution (mL) and m is the mass of the sediments (g). C s is the equilibrium mass of adsorbed substance per unit mass of adsorbent.
Adsorption isotherm models
Adsorption isotherms represent the partitioning of particular species between an aqueous phase and solid particles (sorbate). Adsorption values, over the range of U and Th concentrations studied, were utilized to fit Freundlich, linear and Langmuir models.
Freundlich isotherm
Freundlich isotherm is used for modeling the adsorption on heterogeneous surfaces. This isotherm can be explained by the following equation:
where K f is the Freundlich constant and 1/n is an arbitrary constant called as the adsorption intensity.
The linear form of the Eq. (3) can be written as:
Linear model
If 1/n approaches 1, then K f = K d (partition coefficient) and the equation would be linear.
The linear model can be written as
Langmuir isotherm
Langmuir isotherm model is used for the single coating layer on adsorption surface. This model supposes that the adsorption takes place at a specific adsorption surface. The attraction between molecules decreases as they are getting further from the adsorption surface. Langmuir isotherm can be defined according to the following formulas:
Q 0 and K L are adjustable parameters. As classically defined, Q 0 is the adsorption maxima or monolayer capacity, having the units of C S. The adsorption constant, K L, is a measure of the intensity of the adsorption isotherm (in units of L/kg of adsorbent).
Equation (6) can be written in the following linear form:
Results and discussions
Variation of distribution coefficients (K d) and rate constant (k)
The sorption of U and Th in sediments has been studied by obtaining the value of distribution coefficients (K d) using the batch method. Figure 1 shows the variations of K d with concentration of U and Th spiked in sediment. The K d values varies with the properties of sediment like texture of sediments (sand, silt, clay, loam), organic matter content, pH values, the presence of competing cations, anions and complexing agents. Because of its dependence on many sediment properties, K d values of U and Th in sediment can range over several orders of magnitude under different conditions. Thus, it is very essential to study the geo-chemical characteristics of sediment over a time period. Table 2 shows the mean values of various physico-chemical characteristics of sediments of MHB. All the sediment samples analyzed were dominated by silt and clay. The pH varied to some extent as a result of the different sediment properties but was generally between 7.8 and 8.2.
In this study, K d values have been reported as the mean from two sets of experimental determinations. The measured mean of K d values in sediments for U and Th was found to be 42,140 and 34,256 mL/g, respectively. The resulting coefficient of variation (C v) around mean of K d values for U and Th determined to be 30.53 and 13.62 %, respectively. Subsequently, the rate constants (k) for U and Th in seawater-sediment were determined by assuming the first order of kinetics reaction and observed to be very narrow range of 0.96–1.07 day−1 for U and 0.96–1.02 day−1 for Th. From Fig. 1, initially, at very low concentration, distribution coefficients (K d) follow a linear relationship but generally more complex relationships are observed in which the distribution of species between aqueous and solid phases varies as a function of concentration. After achieving the saturation stage, distribution coefficient observed to be almost constant and at sufficiently high concentration, distribution coefficients decreased because the partitioning behavior deviates from ideality due to removal of species by precipitation.
Statistical analyses
Comparison of means tests was performed on K d and k values for U and Th using single tailed t tests statistics. Under the null hypothesis, there was no statistically significant difference between the means of K d and k values of U and Th obtained in entire experimental sets.
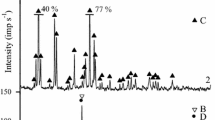
Clay mineralogical studies
Clay mineralogical studies were carried out with the help powder X-ray diffractometer. A typical X-ray diffractogram of MHB sediment is shown in Figs. 2, 3 and 4. The slightly higher K d values for U may be due to the presence of Ca-montmorillonite mineral in sediments which might be responsible for the U sorption because these minerals have high affinity for sorption of U due to exchange with Ca2+ ions in the mineral lattice [4]. Generally, sorption of the U and Th is known to take place primarily as an exchange reaction with metal ions, particularly Ca2+, Na+ and K+ present in the clay minerals such as Ca-montmorillonite, Na-montmorillonite, illite, respectively. The present study determined the Ca-montmorillonite, Na-montmorillonite, talc, calcite, dolomite, quartz, illite and chlorite as predominant minerals in the sediments. Vermiculites and zeolites, are known for their high adsorption capacity for many cations, however, are unable to adsorb relatively high concentration of both Th and U [4].
Adsorption isotherm studies
The results obtained from the experimental studies were applied to Freundlich, linear and Langmuir models as given in Table 3. In present study, all models showed a better fit of the adsorption data due to obtaining very strong degree of the coefficient of determination (R 2 > 0.9) for both U and Th. Therefore we cannot assume that the adsorption behavior fit one of them better and it is a homogeneous or a heterogeneous one. The % adsorption yields were found to be >99 % for both U and Th at the same pH and temperature. In Freundlich model, K f and adsorption intensity (1/n) for U and Th were calculated from the intercepts and slopes of the curve, respectively, and n were found to be very close to unity for both U and Th. Therefore, linear model was observed to be highly suitable. According to the linear model, the distribution coefficient (K d) of Th was found to be about 50 % higher than U indicating that the adsorption of Th is more favorable than U on sediments at the same pH and temperature. This may be due to the decrease of hydration (−ΔH h) and increase of the ionic radius. Th has lower hydration energy (−3,332 kJ/mol) and high ionic radius (1.19 Å) than U (−3,958 kJ/mol, 0.97 Å), which means Th can exchange easily at the sediment surface. Furthermore, Th is more electropositive than U which means that Th binding onto the negative surface and binding strength should be stronger and higher respectively, than U.
The values of K f constant were 95,500 L/kg for U and 23,988 L/kg for Th indicating that sorption of U in sediments were observed to be higher than Th. In contrast, the values for K L constant for Th were about one order of magnitude higher than U. Moreover, Langmuir adsorption maxima for U were 4.5 times greater than Th. The plot of the linear form of all the three models for Th and U adsorption on sediment is shown in Figs. 5, 6, 7, 8, 9 and 10, respectively.
Conclusions
The K d values of U and Th are not only dependent on sediment properties but also on the kinds of minerals in sediments. The results of K d and k values obtained indicated that the sediments have very good sorption properties. Among minerals, Ca bearing minerals such as Ca-montmorillonite, dolomite, calcite in sediments were estimated as predominant minerals in sediments which might be responsible for high U sorption. However, linear model considered that the adsorption of Th is more favorable than U on sediments as found in K L values of Langmuir adsorption model. From the adsorption models, it is very difficult to predict the sediment surface (homogeneous or heterogeneous) because all models showed a better fit of the adsorption data for both U and Th. These types of study can be useful for radionuclides transport and safety assessment models.
References
Choppin GR, Pamela JW (1998) The chemistry of actinide behavior in marine systems. Aquat Geochem 4:77–101
Choppin GR (2007) Actinide speciation in the environment. J Radioanal Nucl Chem 273(3):695–703
Langmuir D (1978) Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochim Cosmochim Acta 42(6):547–569
Syed HS (1999) Comparison studies adsorption of thorium and uranium on pure clay minerals and local Malaysian soil sediments. J Radioanal Nucl Chem 241(1):11–14
Hsi CD, Langmuir D (1985) Adsorption of uranyl onto ferric oxyhydroxides: application of the surface-complexation site-binding model. Geochim Cosmochim Acta 49(9):1931–1941
Sparks DL (1995) Environmental soil chemistry. Academic Press, San Diego
Goldberg S (2002) Competitive adsorption of arsenate and arsenite on oxides and clay minerals. Soil Sci Soc Am J 66:413–421
Acknowledgments
The authors sincerely acknowledge the guidance and help provided by Dr. P. K. Sarkar, Head, Health Physics Division. Thanks are also due to Dr. A. K. Ghosh, Director, H, S and E Group, for constant encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, A., Singhal, R.K., Rout, S. et al. Adsorption and kinetic behavior of uranium and thorium in seawater-sediment system. J Radioanal Nucl Chem 295, 649–656 (2013). https://doi.org/10.1007/s10967-012-1825-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-1825-8