Abstract
Since radiolabeled antibiotics specifically bind to the bacterial components they are promising radiopharmaceuticals for the precise diagnosis and detection of infectious lesions. Doxycycline hyclate (DOX) was chosen to investigate as a new radiolabeled antibacterial agent since its bacteriostatic activity against a wide variety of microorganisms. The aim of the present study is to develop simple and easy formulation of DOX with 99mTc ready to use kit. 99mTc-DOX was developed and standardized under varying conditions of reducing and antioxidant agent concentration, pH, radioactivity dose and reducing agent type. Labeling studies were performed by changing the selected parameters one by one and optimum labeling conditions were determined. After observing the conditions for maximum labeling efficiency and stability, lyophilized freeze dry kits were prepared accordingly. Radiochemical purity was determined with RTLC and RHPLC which was found more than >95 %. Two different freeze dry kits were formulated with optimum labeling conditions. The improved kits were found stable up to 6 months.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The evaluation of diagnostic nuclear medicine can be principally attributed to the existence and chemical versatility of 99mTc-sodium pertecnetate (99mTc) the ideal radiotracer. The nuclear properties of 99mTc consist of its 140 keV gamma photon emission, which is optimum for imaging with gamma cameras used in nuclear medicine. Half-life of 6 h is optimum for preparing the radiopharmaceutical, performing its quality control, and injecting into the patient for imaging studies, yet it is short enough to minimize the absorbed radiation dose [1, 2]. Scintigraphic detection of acute, subacute and chronic infection and inflammation is an important problem in clinical practice, because it may have important implications for the management of patients with infectious or inflammatory diseases. The ideal radiopharmaceutical for infection/inflammation imaging should accumulate efficiently to inflammatory foci, clear rapidly from background tissue, discriminate between bacterial infection and sterile inflammation, cost low, have kit to prepare easily [3]. Since radiolabeled antibiotics specifically bind to the bacterial components they are promising radiopharmaceuticals for the precise diagnosis and detection of infectious lesions [4]. So far various antibiotics studied for infection imaging in nuclear medicine. Some of those are the ciprofloxacin, enrofloxacin, moxifloxacin, kanamycin, lomefloxacin, ofloxacin, cefoperazone, doxorubicin [5–15] were also labeled with 99mTc-pertechnetate and evaluated as infection imaging agents. Also Örümlü [16] has described a radiolabeling procedure for Doxycycline hyclate (DOX) with 99mTc. DOX is a well-known broad-spectrum tetracycline antibiotic obtained by modification of the oxytetracycline molecule. It has bacteriostatic activity against a wide variety of microorganisms, including aerobic and anaerobic Gram-positive and Gram-negative bacteria, chlamydiae, rickettsiae and mycoplasmas. It exerts bacteriostatic effect by inhibiting the bacterial protein synthesis due to the disruption of transfer RNA and messenger RNA at the ribosomal sites [17, 18]. Generator produced 99mTc is the most commonly used radionuclide in nuclear medicine. Availability and affordability of 99mTc and accessibility of single photon emission computed tomography cameras (SPECT) are important preconditions for clinical practice [19–21]. The aim of this study was to prepare 99mTc-DOX in a simple radiochemical method with good labeling efficiency and evaluate the ready to use cold kit formulation thus making it available to the other nuclear medicine centers.
Materials and methods
All chemicals and solvents were of either HPLC or analytical grade and were used without further purification. DOX was obtained from AppliChem (Germany). Stannous tartrate and stannous chloride dehydrate (Stannous chloride) were purchased from Sigma-Aldrich (USA) and ascorbic acid was purchased from Sigma-Aldrich (United Kingdom). 99mTc was eluted from the Molybdenum-99 (99Mo)/99mTc-generator (Nuclear Medicine Department of Ege University). 13 mm Syringe filter with 0.22 μm pore size GH Polypro membrane was purchased from Pall Life Science. All solvents were obtained from Merck (Germany). Radioactive samples counted in a counting unit (Atomlab 100 Dose Calibrator Biodex Medical Systems). Experiments were performed in triplicate unless stated otherwise. Results are reported as mean ± standard error.
Radiolabeling studies
To investigate the optimum conditions, radiolabeling was tested with different types and concentrations of reducing and antioxidant agent. Radiochemical purity (RP) was determined with Radio Thin Layer Chromatography (RTLC) and Radio High Performance Liquid Chromatography (RHPLC) analysis. Two different freeze dry kits were formulated with optimum labeling conditions and stability of kits were performed.
Effect of reducing agent on labeling
99mTc pertecnetate was eluted from 99Mo/99mTc-generator in +7 oxidation state which does not able to label with any compound on direct addition. So prior to labeling procedure, reduction of 99mTc is required for converting 99mTc from the +7 state to a desired lower oxidation state, which can make complexes with the ligand to form the radiopharmaceutical. So far, different types of reduction agents have been used for radiopharmaceuticals, stannous chloride and stannous tartrate is used extensively [22].
To examine the effect of reducing agent concentration on labeling; DOK (1 mg) was dissolved in 0.9 % sodium chloride solution (1 mL). To this stock solution, stannous chloride was added under an atmosphere of bubbling nitrogen. Reduction of 99mTc was performed at acid pH (1 mg reducing agent dissolved in 1 mL 0.1 N HCl) with different amount of stannous chloride (20, 30, 40, 50, 100, 200 and 400 μg). Radiolabeling was performed with 99mTc (37 MBq) in 0.9 % sodium chloride solution (0.1 mL) and solution was allowed to stand at room temperature for 15 min prior to radiochemical analyses.
Also stannous tartrate has been used as reducing agent for radiolabeling studies. DOK (1 mg) was dissolved in 0.9 % sodium chloride solution (1 mL) in adequate number of vials. 20, 30, 40, 50, 100, 200 and 400 μg stannous tartrate (1 mg reducing agent dissolved in 1 mL 0.1 N HCl) was added to each individual vial. Freshly eluted 37 mBq 99mTc was added to each vial. The vials were allowed to stand at room temperature for 15 min prior to radiochemical analyses.
Effect of antioxidant agent on labeling
99mTc radiopharmaceuticals may undergo auto radiolysis during preparation, release, and storage. Decomposition of the radiopharmaceutical prior to or during administration will decrease the targeting capability. Therefore, it is very important to use a stabilizer to minimize the auto radiolysis. Radiolytic stabilizers are often antioxidants, such as ascorbic acid, gentisic acid, and p-aminobenzoic acid [23].
To examine the effect of antioxidant agent on labeling efficiency and stability of the complex, labeling studies were performed in the absence and presence of an antioxidant agent. DOK (1 mg) was dissolved in 0.9 % sodium chloride solution (1 mL) in four groups of vials. 30, 40, 50 and 100 μg stannous chloride was added to each individual group. Each group has three vials and labeling was performed in the absence and presence (0.05 and 0.1 mg) of ascorbic acid. Freshly eluted 37 mBq 99mTc was added to each vial. The vials were shaken for 30 s, filtered through a 0.22 μm pore size membrane filter and incubated for 15 min at room temperature. The labeling efficiency was analyzed by RTLC.
Antioxidant agent effect on labeling efficiency was also performed with the formulations described above by using stannous tartrate instead of stannous chloride.
Effect of incubation time on labeling
After radiolabeling the RP of the complexes were investigated with RTLC studies which performed at different time intervals (5, 15, 30, 45 and 60 min postlabeling).
Effect of pH on labeling
The RP of a 99mTc radiopharmaceutical is highly dependent on the pH of the kit mixture. The effect of the pH on labeling efficiency of 99mTc-DOX was examined for pH 4.75–7.4.
In vitro stability
After labeling DOX with 99mTc, the preparation was left at room temperature for 6 h. The labeling stability of the complex was evaluated by RTLC studies for every hour.
RTLC procedure
Whatman No:1 papers and silica gel coated (SG) plates were used as stationary phases. Free 99mTc was determined by using SG plates as stationary phase and acetone as the mobile phase. Reduced/Hydrolyzed (R/H) 99mTc was determined by Whatman No:1 papers which developed in Acetonitrile/Water/Trifluoroacetic acid (ACN/W/TFA; 50/25/1.5) solvent system. The radioactivity on plates was measured using a TLC scanner (Bioscan AR 2000), and RP % of 99mTc was calculated from the following equation by subtracting from 100 the sum of measured impurities percentages.
Radio high performance liquid chromatography (RHPLC) analysis
The compounds were further analyzed by an Ultra-HPLC system equipped with a C18 column connected to a photodiode array detector (PDA) and additional NaI gamma detector for the 99mTc compounds. The flow rate was 1.0 mL/min for analytical runs. In all runs the eluent was 0.1 % TFA in H2O (solvent A) and 0.1 % TFA in CH3CN (solvent B). For the analytical control and semi preparative separation the method was as follows: 0–2.5 min, 95 % solvent A-5 % solvent B; 2.5–5 min, 50 % solvent A 50 %-solvent B; 5–7.5 min 20 % solvent A 80 %-solvent B; 7.5–10 min 5 % solvent A-95 % solvent B.
Freeze dry kit formulation and radiolabeling
After observing the effect of different parameters on labeling, subsequently two lots were prepared as follows: Lot-A was prepared by mixing 1 mg DOX, 30 μg stannous tartrate and 0.1 mg ascorbic acid. Lot-B was prepared by mixing 1 mg DOX, 30 μg stannous chloride and 0.1 mg ascorbic acid. Both kits solution were filtered through a 0.22 μm pore size filter to glass vials, frozen in a freezer at −80 °C and lyophilized at −20 °C for 24 h.
In vitro radiolabeling studies were performed with 37 mBq 99mTc for the regard to radiation safety of personnel and the environment. Since, human studies with radiopharmaceuticals are performed with higher radiation doses, labeling with freeze dry kits also examined with higher doses of 99mTc ranged between 37 and 370 mBq.
Stability of the freeze dry kits
The kits were stored at +5 ± 3 °C (in a refrigerator) and +25 ± 2 °C/60 % HR ± 5 % (in a stability cabin). The labeling efficiency of the 99mTc-DOX in each kit was checked at different time intervals up to 6 months. The kits were reconstituted with 37 MBq of 99mTc-pertechnetate and the percentage of the radiolabeled drug was determined by RTLC and RHPLC studies.
Statistical analysis
The calculation of means and standard deviations were made on Microsoft Excel. t test was used to determine statistical significance. Differences at the 95 % confidence level (p < 0.05) were considered significant.
Results and discussion
Radiolabeling studies
A new, simple, rapid and efficient direct method for labeling of DOX with 99mTc was developed. Labeling efficiency of the 99mTc-DOX was assessed by both RTLC and RHPLC studies. Two solvent systems were used to distinguish and quantify the amounts of radioactive contaminants (Free 99mTc, R/H 99mTc).
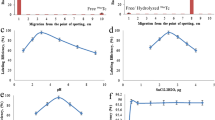
Radiochemical purity and stability of 99mTc-DOX were assessed by RTLC and RHPLC studies. In RTLC using acetone as the solvent, free 99mTc moved with the solvent front, while 99mTc-DOX and R/H 99mTc remained at the spotting point. R/H 99mTc was determined by using ACN/W/TFA (50/25/0.15) as the mobile phase where the R/H 99mTc remained at the point of spotting while free 99mTc and 99mTc-DOX moved with the solvent front. The RTLC chromatogram of 99mTc-DOX was presented in Fig. 1.
The RP of 99mTc-DOK was >95 %, acquired via RTLC and also RHPLC. The RHPLC chromatogram was presented in Fig. 2 and showed two peaks, first one was corresponds to free 99mTc, while the second peak for 99mTc-DOK. The elution times for 99mTc and 99mTc-DOX complex was established as 1.942, 8.873 min respectively.
Effect of reducing agent on labeling
To investigate the effect of reducing agent type on labeling yield, labeling experiments were performed with the same amounts of active ingredient, reducing agent and radionuclide including formulations. Two groups of formulation were prepared. While first group include stannous chloride as reducing agent, second group include stannous tartrate instead. Comparative results for both formulations were shown in Fig. 3.
By increasing the amount of stannous chloride, the labeling efficiency was decreased while the amounts of colloid increased (Fig. 3). By increasing the reducing agent concentration over the optimum values, the labeling yield was slightly decreased. According to radiolabeling studies, no significant differences between groups were assessed.
Effect of antioxidant agent on labeling
In the presence of ascorbic acid stability of the complex was increase slightly while labeling efficiency for early hours was not affected significantly. Increasing of the labeling efficiency takes place due to a decreasing of the R/H 99mTc percentage. The results obtained in these experiments revealed that, maximum RP was obtained with 30 μg stannous chloride, 0.1 mg ascorbic acid and 30 μg stannous tartrate, 0.1 mg ascorbic acid including formulations (Tables 1–8). Effect of incubation time, pH and stability of these formulations were investigated to evaluate the optimum labeling conditions for DOX.
Effect of incubation time on labeling
30 μg stannous chloride, 0.1 mg ascorbic acid and 30 μg stannous tartrate, 0.1 mg ascorbic acid including formulations were labeled with 37 mBq 99mTc. After radiolabeling the RP of the complexes were investigated with RTLC studies which performed at 5, 15, 30, 45 and 60 min postlabeling (Table 9). According to the experiments the RP of the complex was reached over 90 % in 5 min after labeling. Optimal radiolabeling yield was obtained at 15 min incubation period (~95 %) and incubation for longer time intervals did not show any remarkable change.
Effect of pH on labeling
The effect of pH on labeling efficiency was examined for pH 4.75–7.4. According to experiments results the pH of the reaction medium was not found to play an important role in the labeling process (Fig. 4). While keeping other reaction conditions constant and varied the pH of the reaction from 4.75 to 7.4, there is no significant differences on labeling efficiency was observed.
In vitro stability
The complex stability was checked up to 6 h at room temperature. During the incubation period the compound were found stable as determined by RTLC (Fig. 5).
Stability of the freeze dry kits
Kits were labeled with 37, 185 and 370 mBq 99mTc. Slightly decrease in RP was observed with increasing of radioactivity (p < 0.05) (Fig. 6).
The stability of the freeze dry kits were determined at 1, 2, 3, 4, 5 and 6 months after storage both at +5 ± 3 °C (in a refrigerator) and +25 ± 2 °C/60 % HR ± 5 % (in a stability cabin). According to experiments, both kits (Lot-A and Lot-B) were found stable up to 6 months without any significant decrease in labeling yield (Fig. 7) (p > 0.05).
The freeze dry kit preparation of a radiopharmaceutical leads to instant labeling by mixing with 99mTc at the time of use. The reconstituted kit solution was clear and did not contain any visible particle. The freeze dry kits developed in this study were found to be stable with a shelf –life of 6 months when preserved at both at +5 ± 3 °C (in a refrigerator) and +25 ± 2 °C/60 % HR ± 5 % (in a stability cabin). Based on the findings of this study, the complex easily formed by the reconstitution of these kits without any requirement for boiling and post-labeling purification [24].
Conclusion
DOX, is a broad spectrum of activity against a wide range of gram positive and gram negative pathogens [17, 18]. So far various antibiotics were labeled with 99mTc [5]. According to Örümlü’s studies, DOX was labeled with 99mTc [16]. But radiochemical impurities weren’t separated from the complex with RTLC studies. The aim of this study was to standardize and develop a new, simple and ready to use kit of DOX for radiolabeling with 99mTc. 99mTc-DOX was developed and standardized under varying conditions of reducing and antioxidant agent concentration, pH, radioactivity dose and reducing agent type. Labeling studies were performed by changing the selected parameters one by one and optimum labeling conditions were determined. After observing the conditions for maximum labeling efficiency and stability, lyophilized freeze dry kits were prepared accordingly.
Simple method for radiolabeling of DOX with 99mTc has been developed and standardized. Labeling efficiency of 99mTc-DOX was assessed by both RTLC and RHPLC and found higher than 95 %. The resulting complex was quite stable and labeling yield >95 % was maintained for up to 6 h. Two different freeze dry kits was developed and evaluated. Based on the data obtained from this study, both products was stable for 6 months with high labeling efficiency. To examine the role of 99mTc-DOX in imaging of infection at early stage, in vivo studies are in progress.
References
Sharmila B, Maroor R, Ambikalmajan P, Natesan R (2001) Evolution of Tc-99m in diagnosticradiopharmaceuticals. Mumbai. India: radiopharmaceuticals division. Isotope Group, Bhabha Atomic Research Center, Seminars in Nuclear Medicine XXXl (4):266–277
Muhammad J, Irfan UK, Saima M, Ume-Kalsoom D, Syed WH (2012) Synthesis, characterization and biodistribution of novel amine thiophene 99mTc labeled complex. Pak J Pharm Sci 25:381–387
Boerman OC, Dams EM, Oyen WJG, Corstens FHM, Storm G (2001) Radiopharmaceuticals for scintigraphic imaging of infection. Inflamm Res 50:55–64
Seyedeh FM, Mostafa E, Seyed E, Mohammad HT, Farhad HH (2010) Freeze-dried cold kit for preparation of 99mTc-ciprofloxacin as an infection imaging agent. Iran J Nucl Med 18:45–51
Halder KK, Nayak DK, Baishya R, Sarkar BR, Sinha S, Ganguly S, Debnath MC (2011) 99mTc-labeling of ciprofloxacin and nitrofuryl thiosemicarbazone using fac-[99mTc(CO)3(H2O)3] core: evaluation of their efficacy as infection imaging agents. Metallomics 1041–1048
Britton KE, Vinjamuri S, Hall AV, Solanki K, Siraj QH, Bomanji J, Das S (1997) Clinical evaluation of technetium-99m infecton for the localisation of bacterial infection. Eur J Nucl Med 24:553–556
Britton KE, Soroa V, Amaral H (1998) 99mTc “infecton”, preliminary evaluation in over 500 patients through an IAEA coordinate research program. Eur J Nucl Med 25:874
Britton KE, Wareham DW, Das SS, Solanki KK, Amaral H, Bhatnagar A, Katamihardja AH, Malamitsi J, Moustafa HM, Soroa VE, Sundram FX, Padhy AK (2002) Imaging bacterial infection with (99m)Tc-ciprofloxacin (infecton). J Clin Pathol 55(11):817–823
Siaens RH, Rennen HJ, Otto C, Boerman R, Guido S (2004) Synthesis and comparison of 99mTc-enrofloxacin and 99mTc ciprofloxacin. J Nucl Med 45:2088–2094
Sankha C, Sujata SD, Susmita C, Kakali D, Mridula M, Bharat RS, Samarendu S, Shantanu G (2010) Synthesis and evaluation of 99mTc-moxifloxacin, a potential infection specific imaging agent. Appl Radiat Isot 68:314–316
Roohi S, Mustaq A, Jehangir M, Malik SA (2006) Synthesis, quality control and biodistribution of 99mTc-kanamycin. J Radioanal Nucl Chem 267(3):561–566
Motaleb MA (2007) Preparation and biodistrubition of 99mTc-lomefloxacin and 99mTc-ofloxacin complexes. J Radioanal Nucl Chem 272(1):95–99
Motaleb MA (2007) Preparation of 99mTc-Cefoperazone complex, a novel agent for detecting sites of infection. J Radioanal Nucl Chem 272(1):167–171
El-Ghany EA, El-Kolaly MT, Amine AM, El-Sayed AS, Abdel-Gelil F (2005) Synthesis of 99mTc-pefloxacin: a new targeting agent for infectious foci. J Radioanal Nucl Chem 266(1):131–139
Rizvi FA, Bokhari TH, Roohi S, Mustaq A (2012) Direct labeling of doxorubicin with technetium-99m: its optimization, characterization and quality. J Radioanal Nucl Chem 293(1):303–307
Örümlü O (2009) Development of new radiopharmaceuticals fo imaging acute inflammation and infection. PhD Thesis. Ege University, Faculty of Pharmacy
Thawatchai P, Juree C (2008) Antibacterial activity and drug release of chitosan sponge containing doxycycline hyclate. AAPS PharmSciTech 9:829–836
Luis JC, Ana MS, Jose D, Nelida F, Matilde S, Juan JG (2009) Pharmacokinetics of doxycycline in sheep after intravenous and oral administration. Vet J 180:389–395
Saleh TB (2011) Basic sciences of nuclear medicine. Springer, London, pp 10–30
Fred A, Jr M, Milton JG (2012) Radioactivity, radionuclides and radiopharmaceuticals. Essentials of nuclear medicine ımaging. Elsvier Saunders, Philedelphia, pp 1–11
Ahlgren S, Andersson K, Tolmachev V (2010) Kit formulation for 99mTc-labeling of recombinant anti-HER2 affibody molecules with a C-terminally engineered cysteine. Nucl Med Biol 37(5):539–544
Technical Report Series No: 466 (2008) Technetium-99m radiopharmaceuticals: manufacture of kits. International Atomic Energy Agency, Vienna
Liu S (2005) 6-Hydrazinonicotinamide derivatives as bifunctional coupling agents for 99mTc-labeling of small biomolecules. Top Curr Chem 252:117–153
Xiaolin T, Michael JP (2004) Design of freeze-drying processes for pharmaceuticals: practical advice. Pharm Res 21:191–199
Acknowledgments
This study was supported by The Scientific and Technological Research Council of Turkey (Tubitak-110 S 229). The authors would like to acknowledge the support of T.R. Prime Ministry State Planning Organization Foundation Grant Project Number: 09DPT001 for TLC Scanner. Also the authors thank to Ege University Nuclear Medicine Department technicians for their technical assistance for the animal experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
İlem-Özdemir, D., Aşıkoğlu, M. & Özkılıç, H. Radiolabeling, quality control and kit formulation of a new 99mTc-labeled antibiotic: 99mTc-doxycycline hyclate. J Radioanal Nucl Chem 298, 1635–1642 (2013). https://doi.org/10.1007/s10967-013-2547-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-013-2547-2