Abstract
Rapid bioassay methods for 90Sr in urine samples are needed to provide an early estimation of possible internal dose resulting from exposure to radiostrontium in the event of a radiological and nuclear emergency. In this work, a fast column separation method followed by liquid scintillation counting for detection of 90Sr in urine was developed. Replicate spike and blank samples were analyzed for performance evaluation of the method. Using this method, a detection limit of ~10 Bq L−1 for 90Sr can be achieved with a sample analysis turn-around time of 4 h for a set of 12 samples. The method is adequate to meet the radiobioassay acceptance criteria and is suitable for quick dose assessment of 90Sr exposure following a radiation emergency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the event of a radiological/nuclear (RN) emergency, such as a release of radioactive substances during a nuclear power plant accident, local contamination incidents involving misuse of radioactive sources, or illicit dispersion of radioactive material in a radiological dispersal device (RDD) by terrorists, the first responders and public may be internally exposed to radionuclides. Rapid radiobioassay is then needed to provide an early dose assessment so that the physicians can decide if immediate medical intervention is necessary to reduce acute or long-term health effects.
Strontium-90 (half-life of 28.8 years with maximum beta energy of 545.9 keV) is one of the most hazardous radionuclides generated in nuclear operations and nuclear weapon tests, as it is readily incorporated into bone tissues due to its chemical similarity to calcium. Detailed radioecological studies performed after the Chernobyl nuclear accident by the International Atomic Energy Agency (IAEA) have revealed high mobility of radiostrontium in the environment compared to many other radionuclides [1]. As a pure beta emitter with notable commercial uses, 90Sr is also easier to obtain, shield and transport but more difficult to detect during delivery to the target location than a gamma source; thus, it is more likely to be sought by terrorists for a nuclear attack. The U.S. Department of Energy (DOE) and the U.S. Nuclear Regulatory Commission (NRC) interagency working group have identified 90Sr as one of the highest risk radionuclides for the detonation of a RDD by terrorists [2].
In response to a radiation emergency involving internal contamination of 90Sr, a bioassay method for radiostrontium in emergency urine samples with a short analysis turn-around time and a high sample throughput is highly desirable. In the previous study [3], a reference action level of 54 Bq L−1 for 90Sr in emergency urine samples was derived, based on a reference dose to adults of 0.1 Sv committed effective dose equivalent (CEDE). Although several emergency bioassay methods for 90Sr in urine have been recently developed [4–7], most of these methods are inadequate to meet the sensitivity requirements for 0.1 Sv CEDE. In this work, a rapid bioassay method for 90Sr in emergency urine was developed to fill the gaps. Performance tests were carried out to validate the method. Details of this method are described, and the results of the performance tests are reported.
Experimental
Reagents and standards
All the chemicals used in this work were analytical grade or higher and purchased from Sigma-Aldrich Canada (Oakville, Ontario, Canada). The deionized water was obtained from a Millipore Direct-Q5 Ultrapure water system. The extraction resins employed in this work were Sr and TRU Resins (50–100 μm) in 2 mL pre-packed cartridges, available from Eichrom Technologies Inc. (Lisle, Illinois, USA).
The TraceCERT® stable Sr standard (1,000 mg L−1), used as a tracer for monitoring chemical recovery, was purchased from Sigma-Aldrich Canada. Radioisotope standards, including 90Sr, 133Ba, 210Pb, 232U (in equilibrium with its progeny - 228Th), and 242Pu, were obtained from the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA).
Sample preparation
The reagent blank, urine blank and urine spike samples were prepared to examine the performance of the method. The reagent blank sample was prepared by adding 20 mL deionised water to a 50 mL polycarbonate centrifuge tube, while the urine blank sample consisted of an aliquot of 20 mL pooled urine collected from the employees working at the Chalk River Laboratories. The spike sample was prepared by adding known amounts of the 90Sr standard to 20 mL of pooled urine. Prior to analysis, all the blank and spike samples were acidified using concentrated HNO3 to 8 M. To monitor the chemical recovery, 1 mg of the stable Sr carrier was added as tracer for yield correction.
Column separation
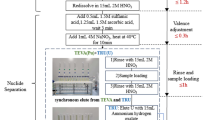
Stacked TRU and Sr resin cartridges were used for column separation of strontium from the urine matrix and other interfering elements. A 12-hole vacuum box (available from Eichrom Technologies Inc.) was used for column separation to increase sample analysis throughput. The Sr resin cartridges were attached to the vacuum box, and the TRU cartridges were inserted on top of the Sr resin columns. Then, 60 mL plastic syringe holders, used as the sample reservoirs, were connected to the top of the Sr resin cartridges using two-way luer-lock valves. Before use, the stacked cartridges were pre-conditioned with 10 mL of deionized water followed by 10 mL 8 M HNO3.
Before loading the sample onto the resins, 1.5 mL of 3 M NaNO2 solution was slowly added to each sample. Once mixed, the sample sat for 10 min to allow for valence adjustment of actinides (e.g., Pu, Th and U) so that these potential interferences could be effectively extracted by the TRU resin and separated from Sr. The sample was passed through the two stacked cartridges at a flow rate of ~2 mL min−1, and Sr was extracted onto the Sr resin. The resins were then rinsed with 10 mL of 8 M HNO3, and the TRU cartridges were removed from the column assembly. After rinsing the Sr resin cartridges with another 20 mL of 8 M HNO3, they were ready for elution.
The Sr was eluted off the Sr resin with 8 mL of 0.05 M HNO3 into a pre-weighed 20 mL plastic scintillation vial. About 0.5 g of the eluate was transferred and diluted with 0.1 M HNO3 to 10 g for analysis of stable Sr by ICP-MS to determine the chemical recovery. The rest of the eluate sample was mixed with 12 mL of Ultima Gold AB liquid scintillation (LS) cocktail (available at PerkinElmer Canada, Woodbridge, Ontario) for liquid scintillation counting (LSC).
Liquid scintillation counting
A Hidex 300SL LS counter (Hidex Oy, Finland) was used for counting of radiostrontium. The sample was counted twice with a counting time of 10 min each: the first count was started immediately after preparation of the final LSC sample, and a repeat count was performed about 8 days later to allow for in-growth of 90Y from its parent isotope 90Sr.
Activity calculation
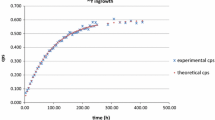
Figure 1 shows the LSC spectra of a spiked urine sample for the first and repeated counting. Although 90Y was in equilibrium with 90Sr when the spike solution was added to the urine sample, it was efficiently removed by column separation due to the low affinity of yttrium onto the Sr resin. Therefore, only the betas from 90Sr (with an endpoint energy βmax = 545.9 keV) were detected for the first counting, which started about 1 h after elution of the Sr resin column. The in-growth factor of 90Y from 90Sr, I Y, can be calculated as follows:
where λ Y and λ Sr are the decay constant of 90Y and 90Sr (s−1); t 0 is the time (in seconds) from the end of column elution to the start of the LSC counting. Meanwhile, the decay factor of 90Sr, D Sr, can be calculated by:
Using the count rate of 90Sr from the first counting immediate after sample preparation, the beta activity of 90Sr, A Sr (Bq), can be calculated by the following equation:
where CRS is the sample count rate in the default region of interest (ROI) for the 90Sr–90Y counting window, which was set at approximate beta energies from 50 to 2,500 keV as shown in Fig. 1; while CRb is the background count rate in the 90Sr–90Y ROI of a blank sample prepared by mixing 7.5 mL deionized water with 12 mL of Ultima Gold AB LS cocktail; R is the chemical recovery, which was determined by the ratio of stable Sr found in the eluate sample by ICP-MS to the total amount of the Sr tracer added to the urine (or blank) sample; ε S and ε b are respectively the sample and background counting efficiencies, which were determined based on experimental triple-to-double-coincidence ratio (TDCR) for individual counting sample; f is the weight fraction of the eluate transferred to the final LSC sample.
Re-counting of the sample source with a delay relative to the time of Sr–Y column separation would result in the in-growth of 90Y daughter, and the 90Sr activity, \( A^{\prime}_{\text{sr}} \) (Bq), can be calculated according to the equation below:
where \( {\text{CR}}^{\prime}_{\text{s}} \) and \( {\text{CR}}^{\prime}_{\text{b}} \) are the sample and background count rates respectively from re-counting; \( \varepsilon^{\prime}_{\text{s}} \) and \( \varepsilon^{\prime}_{\text{b}} \)are the sample and background counting efficiencies respectively for re-counting; while \( D^{\prime}_{\text{sr}} \) and \( I^{\prime}_{\text{y}} \) are the decay factor of 90Sr and the in-growth factor of 90Y at the starting time of re-counting, respectively.
Results and discussion
Two set of samples were prepared and analyzed to evaluate the performance of the method. Each set of samples included 4 reagent (procedural) blanks, two urine blanks, and six urine samples spiked with a known amount of 90Sr at a concentration level of approximately 30 or 300 Bq L−1.
Procedural blanks and minimal detectable activity
The results for the reagent blank samples are given in Table 1. The average chemical recovery for eight reagent blanks was found to be 82 ± 4 %. The mean procedural blank was measured to be −0.004 ± 0.02 Bq for immediate counting and −0.005 ± 0.07 Bq for 90Y in-growth counting. Four urine blanks were also analyzed, and the results are listed in Table 2. No observable 90Sr activity was found in these urine blank samples.
Using the Currie equation [8], the minimal detectable activity (MDA) can be calculated as follows:
where k = 1.645 for a 95 % confidence interval; B is the background counts in the default 90Sr–90Y ROI; T is the counting time (s); and ε is the mean counting efficiency for all the samples counted in this study, which was found to be 95.0 ± 4.0 %. The MDAs of all the reagent blank samples were averaged to be 0.21 ± 0.02 Bq. Given that 20 mL of urine sample was used for the assay, the present method has a detection limit of ~10 Bq L−1, which is adequate to detect the action level of 54 Bq L−1 of 90Sr in urine to meet the sensitivity requirement for the emergency action dose threshold of 0.1 Sv CEDE [3].
Performance evaluation for spiked urine samples
The results for the urine samples spiked with known amounts of 90Sr at concentration levels of ~30 and 300 Bq L−1 are summarized in Tables 3 and 4, respectively. The measurements for all the urine spike samples agreed very well with the expected values. To evaluate the performance of the method, the relative bias and relative precision were also calculated according to the guidance provided by ANSI/HPS N13.30 [9]. The overall relative biases were found to be within ± 3 % and the relative precisions were <12 %, which easily met the radiobioassay acceptance criteria as specified by ANSI/HPS N13.30 (i.e., −25 % to +50 for relative bias; and ± 40 % for relative precision). In addition, the chemical recoveries for all the tested urine blank and spike samples (see Tables 2, 3, 4) were found to be fairly consistent with a variation from 73 to 93 % and an average of 85.1 ± 5.1 %.
Potential interferences
In 8 M HNO3, Pu(IV), Np(IV), Pb(II), Ba(II), Th(IV) and U(VI) will also be extracted onto the Sr resin [10]. The beta- or alpha-emitting isotopes of these elements, if present in the urine sample at a significantly high concentration, could potentially interfere with the 90Sr–90Y detection by LSC, as the default ROI analysis window was set wide open from 50 to 2,500 keV. However, under normal circumstances, most of these radionuclides (except for 133Ba, 210Pb and 241Pu) are unlikely to be present in urine at a high enough concentration level to cause any noticeable interference to the 90Sr–90Y counting. As a low energy beta emitter, 241Pu (with an endpoint energy βmax = 20.8 keV) would not interfere as it is excluded from the default ROI window set for 90Sr–90Y. Furthermore, insertion of a TRU resin cartridge in the column separation step ensures complete removal of any actinides (if present in the urine sample) before they reach the Sr resin. Other resin cartridges, including anion exchange resin AGMP-1, Eichrom TEVA, UTEVA and DGA resins, could also be used for separation of actinides and strontium [7, 11]. In order to examine decontamination of the potential inferences, urine samples spiked with known activities of 133Ba, 210Pb, 242Pu, and 232U/228Th were followed through the entire procedure. The activity in the eluate sample of the Sr resin was then measured by gamma spectrometry for 133Ba, by LSC for 210Pb, and by alpha spectrometry for 242Pu, 232U and 228Th. The decontamination factors of these interfering radionuclides were calculated as the ratio of the activity found in the eluate to the total activity spiked, and the results are summarized in Table 5. High decontamination factors were found for all the radionuclides tested in this study, demonstrating excellent separation of Ba, Pb, Pu, U and Th from Sr using the present Sr urinalysis procedure.
Other radiostrontium isotopes present in the sample, such as 89Sr (β- decay; half-life: 50.57 days; βmax = 1,495.1 keV) and 85Sr (electron capture; half-life: 64.85 days; decay energy QEC2 = 551 keV, 99.2 %; QEC3 = 1,065 keV, 0.8 %), could also interfere with the analysis of 90Sr by LSC. Although a difference in the LSC spectrum may be noticeable, this interference is more complicated to deal with as these Sr isotopes cannot be chemically separated from 90Sr. Since 85Sr is detected by LSC through the emission of low energy Auger electrons and X-rays following the electron capture process, its contribution to the LSC spectrum is mainly below 20 keV in the LS energy scale and tailing of its spectral peak to the background in the default 90Sr–90Y counting window (50–2,500 keV) is expected to be minimal. Thus, the interference of 85Sr is much less a concern. Even if 85Sr is present at a higher concentration in the urine sample, its activity concentration can be quantified directly by the LSC measurement using a low energy window (e.g., 3–40 keV) or via additional gamma spectrometry counting utilizing its gamma emission of a 514 keV spectral line. The measured 85Sr activity can then be used for correction of its interference to the assay results of 90Sr in the sample. Any 89Sr, if present in the sample, can also be deduced by the difference between the first counting activity A Sr from Eq. (3) and the 90Y in-growth counting activity \( A^{\prime}_{\text{sr}} \) from Eq. (4) with decay correction of 89Sr for the time from the first counting to re-counting. It should be noted that, regardless of the presence of 85Sr and 89Sr in the sample, the 90Sr activity (i.e., \( A^{\prime}_{\text{sr}} \) ) determined from in-growth activity of 90Y would remain unchanged. In the case that both 90Sr and 89Sr are present in the sample and the differentiation of 89/90Sr is needed for immediate reporting, Cerenkov counting techniques as described in references [12, 13] can also be used for a direct measurement of 89Sr in the eluate sample obtained from the present column separation procedure.
Sample analysis throughput and turn-around time
The present strontium bioassay procedure is very fast and easy to operate. Using a 12-hole vacuum box system, it takes about one and half hours to process a batch of 12 samples through the entire chemical separation steps from the sample acidification to the LSC sample preparation. In the event of an RN emergency that a more rapid dose assessment is required, the first counting results of radiostrontium activity can be reported within a total turn-around time of 4 h, while the 90Y in-growth results by re-counting can be reported a week later for confirmation.
Conclusions
A rapid bioassay method has been developed for the determination of radiostrontium in emergency urine samples. The method involves column separation of Sr from a small volume (20 mL) of urine using stacked Eichrom TRU and Sr resin with a vacuum box system for fast analysis turn-around time and high sample throughput. The Sr activity was measured by LSC using a Hidex TDCR LS counter, and a detection limit of 10 Bq L−1 for 90Sr has been achieved for a counting time of 10 min. Urine samples spiked with known amounts of 90Sr were tested for performance evaluation of the method. Satisfactory accuracy and precision has been achieved. The method meets the requirements for sensitivity, accuracy and repeatability for emergency bioassay for 90Sr in urine if a committed effective dose equivalent of 0.1 Sv is used as the action dose threshold for medical intervention.
References
International Atomic Energy Agency (1992) Assessment of radiological consequences and evaluation of protective measures, The International Chernobyl Project, Technical report of the IAEA
U.S. Department of Energy/U.S. Nuclear Regulatory Commission (DOE/NRC) (2003) Radiological dispersive devices: an initial study to identify radioactive materials of greatest concern and approaches to their tracking, tagging, and disposition. Prepared by the DOE/NRC Interagency Working Group on Radiological Dispersal Devices. http://energy.gov/sites/prod/files/edg/media/RDDRPTF14MAYa.pdf. Accessed 16 July 2012
Li C, Vlahovich S, Dai X, Richardson RB, Daka JN, Kramer GH (2010) Health Phys 99:702–707
Li C, Sadi BB, Moodie G, Daka JN, Lai EPC, Kramer GH (2009) Radiat Prot Dosim 136:82–86
Sadi BB, Li C, Jodayree S, Lai EPC, Kochermin V, Kramer GH (2010) Radiat Prot Dosim 140:41–48
Plionis A, Gonzales ER, Landsberger S, Peterson D (2009) Appl Radiat Isot 67:14–20
Maxwell SL, Culligan BK (2009) J Radioanal Nucl Chem 279:105–111
Currie LA (1968) Anal Chem 40:586–593
Health Physics Society (1996) Performance criteria for radiobioassay: an American National Standard. American National Standard Institute, ANSI/HPS N13.30, New York
Horwitz EP, Chiarizia R, Dietz ML (1992) Solvent Extr Ion Extr 10:313–336
Dai X, Kramer-Tremblay S (2011) J Radioanal Nucl Chem 289:461–466
Popov L, Hou X, Nielsen SP, Yu Y, Djingova R, Kuleff I (2006) J Radioanal Nucl Chem 269:161–173
Chu TC, Wang JJ, Lin YM (1998) Appl Radiat Isot 49:1671–1675
Acknowledgments
This work was supported by the CRTI 08-241TD project (Field Techniques for Emergency Radiobioassay).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, X., Cui, Y. & Kramer-Tremblay, S. A rapid method for determining strontium-90 in urine samples. J Radioanal Nucl Chem 296, 363–368 (2013). https://doi.org/10.1007/s10967-012-1971-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-1971-z