Abstract
The unsymmetrical diglycolamides (DGAs) such as N,N-dihexyl-N′,N′-dioctyl-3-oxapentane-1,5-diamide (DHDODGA), N,N-didecyl-N′,N′-dioctyl-3-oxapentane-1,5-diamide (D2DODGA), N,N-didodecyl-N′,N′-dioctyl-3-oxapentane-1,5-diamide (D3DODGA), were synthesized, and characterized by IR, NMR, and mass spectroscopic techniques. The extraction behaviour of Am(III), Eu(III), and Sr(II) by the solutions of these unsymmetrical DGAs in n-dodecane was studied as a function of concentration of nitric acid and DGA. The distribution ratio of Am(III) and Eu(III) increased with increase in the concentration of nitric acid; whereas, the distribution ratio of Sr(II) reached a maximum at 4 M nitric acid followed by decrease at higher acidities. The extraction of Am(III) and Eu(III) in 0.1 M DGA/n-dodecane decreased in the order DHDODGA > D2DODGA > D3DODGA. However, the order changed upon lowering the concentration of DGA. The third-phase formation behaviour of nitric acid and neodymium(III) in 0.1 M DGA/n-dodecane was studied as a function of concentration of nitric acid. The limiting organic concentration of nitric acid and neodymium increased with increase in the chain length of alkyl group attached to amidic nitrogen. Near stoichiometric amount of neodymium(III) was loaded in 0.1 M D3DODGA/n-dodecane without the formation of third-phase from 3 to 4 M nitric acid medium. The study revealed that the unsymmetrical diglycolamides D2DODGA and D3DODGA are superior candidates for partitioning the minor actinides from high-level liquid waste.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diglycolamides (DGAs) are alkyl-3-oxapentane-1,5-diamide derivatives, emerging as promising candidates for the separation of trivalent actinides and lanthanides from high-level liquid waste (HLLW) [1–4]. The presence of an etheric oxygen between the two-amide groups in DGAs increases the extraction of trivalents due to enhanced tridentate complex formation [3]. The DGAs studied for partitioning of minor actinides so far are, in general, symmetrical i.e. the alkyl groups attached to both the amidic nitrogen atoms are structurally identical. It is realized that the extraction and stripping behavior of trivalents are strongly dependent on the nature of alkyl group attached to the nitrogen atom. Therefore, the separations achieved using DGAs are unique to the properties of alkyl substituents [1, 5].
Sasaki et al. [1] studied the solubility of various symmetrical DGAs in aqueous phase as well as in diluents such as n-hexane and n-dodecane. It was reported that the solubility of DGAs in aqueous phase decreased and that in n-dodecane increased with increase of alkyl chain length attached to amidic nitrogen atom. When the number of carbon atoms in the alkyl chain was 8 or more, the solubility of DGA in aqueous phase was quite low. In addition, the authors also studied the extraction behaviour of Eu(III) and Am(III) in various symmetrical DGAs. The distribution ratio of these trivalent metal ions suffered a notable decrease with increase in the chain length of alkyl group. Based on those studies, the octyl derivatives such as N,N,N′,N′-tetraoctyldiglycolamide (TODGA) [4–7], and N,N,N′,N′-tetra-2-ethylhexyldiglycolamide (TEHDGA) [8, 9] were chosen as promising candidates for the extraction of trivalent actinides from nitric acid medium. However, these DGAs required the phase modifiers such as tri-butylphosphate (TBP), N,N-dihexyl-octanamide (DHOA) or long chain alcohols to avoid the third-phase formation. Thus, Magnusson et al. [7] demonstrated the extraction and the recovery of minor actinides from the real PUREX raffinate using a solution of TODGA-TBP in hydrogenated tetrapropylene (TPH). Tachimori et al. [10] proposed a monoamide based phase modifier, DHOA, for the TODGA/n-dodecane system. Similarly, iso-decanol and N,N-dihexyloctamide (DHOA) were used by several authors as phase modifier in TEHDGA/n-dodecane system [8, 9].
Third-phase formation is a splitting of organic phase, during the course of extraction, into two phases with the heavier one, rich in metal-solvate and the lighter phase rich in diluent. Usually, it occurs due to the poor solubility of the polar metal-solvate complex in a non-polar diluent phase at metal loadings beyond a particular value referred as limiting organic concentration (LOC). The corresponding aqueous phase concentration of metal ion is called as critical aqueous concentration (CAC) [11]. Several parameters such as temperature, aqueous phase acidity, structure of the extractant, and nature of diluent etc., can influence third-phase formation [12]. Sasaki et al. [13] studied the third-phase formation of neodymium(III) in symmetrical DGAs. The LOC of neodymium(III) increased with increase in the concentration of DGA and decreasing the concentration of HNO3. In the presence of phase modifier, DHOA, the LOC of neodymium increased with increase in the concentration of DHOA. Interestingly, the authors also reported that the third-phase was not formed in tetradodecyldiglycolamide/n-dodecane, (TDdDGA/n-dodecane) system during the loading of neodymium(III) from 3 M nitric acid. The stoichiometry of Nd:TDdDGA is as high as 1:3 was reported in this system. Despite the absence of third-phase in TDdDGA system, several authors employed a solution of TODGA along with a phase modifier in n-dodecane, for the separation of trivalent actinides from nitric acid medium. This was in fact due to the poor distribution ratio of trivalents obtained in TDdDGA/n-dodecane (<10 at 1 M HNO3) as compared to the TODGA system.
In the recent past, unsymmetrical DGAs are being studied for the extraction of trivalent actinides from nitric acid medium [14]. In unsymmetrical DGAs, the alkyl groups attached to amidic nitrogen atom are structurally different. Recently, we prepared diethylhexyl-dioctyl-diglycolamide (DEHDODGA), and studied for the extraction of some actinides, and fission products from nitric acid medium [14]. Our findings showed that the separation factor of americium over strontium achieved with the use of DEHDODGA was higher than the corresponding symmetrical DGAs. Considering the advantage of n-octyl group in enhancing the distribution ratio and n-dodecyl group in avoiding third-phase formation, the present study, deals with the synthesis of various unsymmetrical DGAs with n-octyl group in one arm of DGA, and the alkyl group varied from hexyl to dodecyl at the other arm of DGA. The study was carried out to identify the superior unsymmetrical diglycolamide that exhibits efficient extraction of trivalents without leading to any third-phase formation. The extraction behavior of Am(III), Eu(III), and Sr(II) from nitric acid medium in a solution of these unsymmetrical DGAs in n-dodecane was studied as a function of various parameters. The third-phase formation behaviour of nitric acid and neodymium(III) was studied at various concentrations of nitric acid in 0.1 M DGA/n-dodecane and the results are reported in this paper.
Experimental
Materials
All the chemicals and reagents used in this study were of analytical grade. (152+154)Eu(III), 241Am(III), and (85+89)Sr(II) were procured from Board of Radiation and Isotope Technology, Mumbai, India. Neodymium nitrate was purchased from Alfa Aesar, Mumbai, India. Diglycolic anhydride, dihexylamine, dioctylamine, and didecylamine were procured from Aldrich. Didodecylamine was obtained from Fluka. Bruker AVANCEIII 500 mHz was used for NMR analysis. IR spectra were recorded using Bomem FTIR spectrometer model-103. A home built reflectron time of flight mass spectrometer and Thermoscientific DSQ II Thermo fisher scientific electron ionization mass spectrometer coupled with gas-chromatography were used for mass analysis.
Synthesis of DGAs
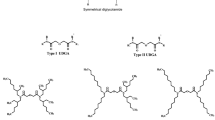
The procedure for the synthesis of symmetrical DGAs is described elsewhere [15]. A similar procedure was adopted for the synthesis of unsymmetrical DGAs [14]. Briefly, the first step involved the reaction between diglycolic anhydride and dialkylamine to obtain diglycolamic acid. In the second step, the diglycolamic acid was reacted with another (desired) dialkylamine in the presence of dicyclohexylcarbodiimide (DCC) to obtain the unsymmetrical diglycolamide. The reaction scheme for the synthesis of a typical unsymmetrical DGA is shown in Scheme 1. The structures of various diglycolamic acids and DGAs studied in the present work are shown in Fig. 1. The details of synthesis are given below.
The diglycolamic acids, dioctyldiglycolamic acid (DODGAA) and didodecyldiglycolamic acid (D3DGAA) were synthesised by the procedure (step 1 in the Scheme 1) described elsewhere [14]. The product obtained was washed extensively with distilled water followed by ~1 M HCl, and distilled water. This procedure was repeated several times to remove the unreacted anhydride or dialkyl amine, if any. The organic phase was dried with anhydrous sodium sulphate and the solvent was removed under vacuum. The residue was recrystallised from hexane to obtain the white coloured powders of DODGAA or D3DGAA. The product was then characterized by IR, NMR, and mass spectroscopic techniques. The yield was >90 % in both the cases and the purity of the compounds was >98 %.
The NMR, FTIR, and mass characterization details are provided below. DODGAA: 1H-NMR (500 mHz, CDCl3, TMS, 298 K), δ 0.89 (t, 6H, N–(CH2)7–CH 3), 1.29, 1.55 (s, 24 H, –N–CH2–(CH 2)6–CH3), 3.10, 3.35 (t, 4 H, –N–CH 2–(CH2)6–CH3), 4.21 (s, 2H, –N–CO–CH 2–O–), 4.38 (s, 2H, –CH 2–COOH), 9.34 (s, 1H, –COOH). 13C-NMR (500 mHz, CDCl3, TMS, 298 K) δ 171.93, 170.42, 77.29, 77.05, 72.63, 70.98, 46.88, 46.79, 31.75, 31.71, 29.18, 29.17, 29.11, 29.06, 28.62, 26.92, 26.87, 25.89, 22.59, 22.57. The IR spectrum showed the transmittance bands at the following frequencies (cm−1) 3413 (broad, O–H stretching), 2926 (–CH3-stretching), 1713 (C=O-stretching), 1628 (C=O-stretching), 1466 (CH2-bending), 1400 (–C–N- stretching), 1375 (–CH3-bending), 1120 (C–O-stretching). The molecular ion peak in the mass spectrum was found at m/z 357.4.
D3DGAA: 1H-NMR (500 mHz, CDCl3, TMS, 298 K), δ 0.89 (t, 6H, N–(CH2)11–CH 3), 1.27, 1.56 (s, 40 H, –N–CH2–(CH 2)10–CH3), 3.11, 3.36 (t, 4 H, –N–CH 2–(CH2)10–CH3), 4.22 (s, 2H, –N–CO–CH 2–O–), 4.40 (s, 2H, –CH 2–COOH). 13C-NMR (500 mHz, CDCl3, TMS, 298 K) δ 170.59, 77.28, 77.03, 76.78, 71.17, 46.88, 31.90, 29.61, 29.58, 29.52, 29.50, 29.33, 29.31, 29.23, 28.62, 27.41, 26.94, 26.8, 22.67, 14.09. The IR spectrum showed the transmittance bands at the following frequencies (cm−1) 3424 (broad, O–H stretching) 2918, 2849 (–CH3-stretching), 1749 (C=O-stretching), 1598 (C=O-stretching), 1465 (CH2-bending), 1355 (–C–N- stretching), 1360 (–CH3-bending), 1126 (C–O-stretching). The molecular ion peak in the mass spectrum was found at m/z 468.6.
The procedure for the synthesis of unsymmetrical diglycolamide from diglycolamic acid (step 2 of Scheme 1) is described elsewhere [14]. The product was purified by column chromatography using silica gel. The compound was characterized by NMR, IR, and mass spectral techniques.
The unsymmetrical DGAs, N,N-dihexyl-N′,N′-dioctyl-3-oxapentane-1,5-diamide (DHDODGA), N,N-didecyl-N′,N′-dioctyl-3-oxapentane-1,5-diamide (D2DODGA) were synthesised by the procedure described above. However, the synthesis of N,N-didodecyl-N′,N′-dioctyl-3-oxapentane-1,5-diamide (D3DODGA) involved the initial preparation of D3DGAA (rather than DODGAA) by the procedure discussed above. The product (D3DGAA) was then reacted with dioctylamine to obtain D3DODGA. This variation was due to the relatively poor reactivity of didodecylamine with DODGAA in step 2.
The NMR, FTIR, and mass characterization details are provided below. DHDODGA: 1H-NMR (500 mHz, CDCl3, TMS, 298 K) at δ 4.34 (s, 2H), –O–CH 2–CO–N–, 4.30 (s, 2H), –O–CH 2–CO–N–, 3.31–3.24 (m, 4 H), –CO–N–CH 2–R, 3.20–3.11 (m, 4 H)- –CO–N–CH 2–R, 1.52 (m, 8 H), CH3–CH 2–(CH2) n –N–, 1.33–1.28 (m, 32 H) CH3–CH2–(CH 2) n –CH2–N–, 0.89–0.86 (m, 12 H) CH 3–(CH2) n –N. 13C-NMR (500 mHz, CDCl3, TMS, 298 K) at δ 169.04, 168.43, 69.07, 68.48, 50.08, 47.27, 46.90, 45.75, 34.04, 32.24, 31.80, 30.56, 29.37, 29.33, 29.28, 29.20, 29.16, 28.93, 28.78, 28.55, 27.56, 27.47, 27.00, 26.97, 26.86, 26.66, 25.71, 25.32, 25.01, 24.69, 22.59, 14.02, 13.96. The IR spectrum showed the transmittance bands at the following frequencies (cm−1) 2928, 2851 (C–H stretching), 1654 (C=O-stretching), 1376 (C–N stretching), 1120 (C–O-stretching). The molecular ion peak in the mass spectrum of DHDODGA was found at m/z 524.3. Yield was >65 % and the purity was >98 %.
D2DODGA: 1H-NMR (500 mHz, CDCl3, TMS, 298 K) at δ 4.35 (s, 2H) –O–CH 2–CO–N–, 4.309 (s, 2H) –O–CH 2–CO–N–, 3.20–3.17 (m, 4H) –CO–N–CH 2–R, 3.19–3.11 (m, 4 H) –CO–N–CH 2–, 1.54–1.51 (m, 8 H) CH3–CH2–(CH2) n –CH 2–N–, 1.28–1.27 (m, 48 H) CH3–CH2–(CH 2 ) n –CH2–N, 0.89–0.87 (m, 12 H) CH 3–(CH2) n –N. 13C-NMR (500 mHz, CDCl3, TMS, 298 K) δ 169.08, 168.47, 77.29, 77.04, 76.79, 71.59, 69.09, 68.48, 54.61, 50.09, 47.03, 46.93, 46.22, 45.78, 32.29, 31.89, 31.88, 31.83, 31.80, 31.74, 30.50, 29.58, 29.55, 29.51, 29.41, 29.37, 29.32, 29.30, 29.24, 29.20, 28.96, 28.80, 27.59, 27.0, 26.87, 26.09, 25.4, 24.7, 22.6, 14.75. The IR spectrum showed the transmittance bands at the following frequencies (cm−1) 2921, 2859 (C–H stretching), 1654 (C=O-stretching), 1355 (C–N stretching), 1112 (C–O-stretching). The molecular ion peak in the mass spectrum was found at m/z 637.8. Yield was >65 % and the purity of the compound was >98 %.
D3DODGA: 1H-NMR (500 mHz, CDCl3, TMS, 298 K) at δ 4.35 (s, 2H), –O–CH 2–CO–N–, 4.17 (s, 2H), –O–CH 2–CO–N–, 3.28–3.25 (m, 4 H), –CO–N–CH 2–R, 3.15–3.12 (m, 4 H) –CO–N–CH 2–R, 1.56–1.51 (m, 8 H) CH3–CH 2–(CH2) n –N–, 1.27–1.27 (m, 56 H) CH3–CH2–(CH 2) n –CH2–N–, 0.90–0.88 (m, 12 H) CH 3–(CH2) n –N. 13C-NMR (500 mHz, CDCl3, TMS, 298 K) δ 169.11, 167.79, 153.57, 77.03, 76.78, 71.59, 69.09, 68.49, 54.62, 50.11, 47.05, 46.94, 45.84, 45.79, 32.29, 31.91, 30.51, 29.64, 29.57, 29.55, 29.50, 29.47, 29.43, 29.39, 29.35, 29.30, 28.99, 28.97, 27.60,27.52,27.05, 27.02, 26.90, 26.84, 26.50, 26.09, 25.52, 25.34,24.72, 23.86, 22.68, 20.81, 17.49, 14.11. The IR spectrum showed the transmittance bands at the following frequencies (cm−1) 2924 (C–H stretching), 1651 (C=O-stretching), 1350 (C–N stretching), 1126 (C–O-stretching). The molecular ion peak in the mass spectrum was fond at m/z 692.7. Yield was >60 % and the purity was >98 %.
Extraction of metal ions
All the extraction experiments were conducted in duplicate with 1:1 aqueous: organic phase ratio at 298 K unless otherwise mentioned. The organic phase was composed of a desired concentration of DGA in n-dodecane and the aqueous phase was nitric acid (10−3–8 M). The organic phase was pre-equilibrated with desired concentration of nitric acid. The extraction experiments involved equilibration of equal volumes (1 mL) of organic phase and nitric acid solution spiked with radioisotopes such as 241Am(III) or (152+154)Eu(III) or (85+89)Sr(II), for about 1 h. The distribution ratio of the metal ions was determined by measuring the radioactivity of these isotopes in aqueous and organic phases using a well-type NaI(Tl) scintillation detector, and using Eq. (1).
where M is 241Am(III) or (152+154)Eu(III) or (85+89)Sr(II).
Nitric acid extraction
Extraction of nitric acid by organic phase was carried out by equilibrating equal volumes of organic phase with 1 M nitric acid for about 1 h at 298 K. The concentration of DGA in organic phase was varied from 0.01 to 0.5 M. The concentration of nitric acid present in organic phase was determined by titrating a known volume of organic phase with standard sodium hydroxide in the presence of methanol water mixture. The conditional acid extraction constant (K H ) of the extractant was computed from the extraction data by the procedure described elsewhere [14].
Third-phase formation studies
The studies related to third-phase formation were carried out by the procedure described elsewhere [16, 17]. The procedure is briefly described here. The organic phase was composed of 0.1 M DGA in n-dodecane and the aqueous phase was the solution of desired concentration of neodymium nitrate in desired concentration of nitric acid. The organic phase was pre-equilibrated twice with desired concentration of nitric acid and separated from aqueous phase. The experimental setup for the third-phase formation studies consisted of an equilibration tube immersed in a double walled glass container. The temperature of the container was maintained at a desired temperature by circulating the water from a constant temperature water bath. The equilibration was carried out by mixing equal volumes (1 mL) of doubly pre-equilibrated organic phase with aqueous phase containing neodymium(III) nitrate in nitric acid at a fixed temperature maintained by a constant temperature water bath. The concentration of the nitric acid in this case was similar to that used for pre-equilibration. The mixing was carried out by using a magnetic stirrer. The third-phase formed, was then carefully dissolved by drop-wise addition of the doubly pre-equilibrated organic phase. The concentrations of neodymium in organic and aqueous phases were determined by complexometric titrations using standard EDTA solution to obtain the LOC and CAC respectively. The procedure involved titration of a known volume of aliquot, taken in a methanol water mixture, with standard EDTA solution using methyl thymol blue as indicator. To determine the LOC and CAC of nitric acid, the organic phase was equilibrated with nitric acid until the third-phase was formed. It was then dissolved by the addition of distilled water. The concentration of nitric acid present in organic and aqueous phases was determined as described above (in the “Nitric acid extraction” section).
Results and discussion
Nitric acid extraction and K H determination
The “conditional acid extraction constant” of the ligand (K H ) is an important parameter that decides the efficiency of metal ion extraction. In DGAs, the magnitude of K H depends upon the nature of alkyl group attached to amidic nitrogen atom. The K H values of unsymmetrical DGAs were estimated by the procedure described elsewhere [14]. Makoto Arisaka et al. [18] reported a K H value of 0.38 for TODGA, Deepika et al. [19] reported a K H value of 0.19 for TEHDGA at 298 K. The interaction of nitric acid with DGA is given by equilibrium expression 2.
where the subscripts (aq) and (org) denote the aqueous and organic phases respectively. The “conditional acid extraction constant” (K H ) can be related to the nitric acid concentration in organic phase, as
where, [DGA](org. free) = [DGA]initial − [H+](org) and [H+](org) = [HNO3.nDGA](org).
From Eq. (3), the plot of {log[H+](org) − 2log[H+](aq)} against log[DGA](org.free) results in a straight line. The slope, n, gives the number of molecules of DGA involved in the formation of an adduct with HNO3, and the intercept (log K H ) gives the conditional acid extraction constant of the ligand. The plot of {log[H+](org) − 2log[H+](aq)} against log[DGA](org. free) is shown in Fig. 2. From the intercept, the K H values were determined to be 0.08, 0.12, and 0.07 for DHDODGA, D2DODGA, and D3DODGA respectively. Excluding DHDODGA, the magnitude of K H decreases with increase in the chain length of alkyl group in the order TODGA [18] (0.38) > D2DODGA (0.12) > D3DODGA (0.07). This indicates that the conditional acid extraction constant of DGA decreases with increase of alkyl chain length attached to amidic nitrogen atom, perhaps due to the steric hindrance during protonation. The deviation observed for DHDODGA is not clear at present.
Extraction of metal ions
The variation in the distribution ratio of Am(III) as a function of nitric acid concentration in unsymmetrical DGAs/n-dodecane is shown in Fig. 3. It is observed that the distribution ratio increases with increase in the concentration of nitric acid as expected for the DGA systems. A distribution ratio of >300 is obtained in 0.1 M D2DODGA/n-dodecane, and 0.1 M D3DODGA/n-dodecane systems when the concentration of nitric acid is varied from 4 to 7 M. The distribution ratio of Am(III) in 0.1 M DHDODGA/n-dodecane could not be measured above 4 M nitric acid due to its third-phase formation. It is interesting to observe from Fig. 3, that the distribution ratio of Am(III) decreases in the order DHDODGA > D2DODGA > D3DODGA. The distribution ratio of Am(III) in 0.1 M TODGA/n-dodecane [4] is comparable with the distribution ratio obtained in 0.1 M DHDODGA/n-dodecane. A similar trend is also observed for the extraction of Eu(III), shown in Fig. 4. However, the distribution ratio of Eu(III) is always higher than Am(III) at all acidities in all DGAs investigated. A similar observation was also reported by others in symmetrical DGA systems [4, 9]. Sasaki et al. [1] reported a decrease in the distribution ratio of trivalent actinides with increase in the chain length of alkyl group attached to amidic nitrogen atom. However, Mowfy et al. [5] reported some deviations from the expected extraction trend. For instance, the extraction of Am(III) in symmetrical DGAs in benzene increases when the alkyl group attached to amidic nitrogen was varied in the order octyl < hexyl < propyl < butyl. However, the reason for such behaviour was not reported. Nevertheless, the studies on DGAs carried out so far, in general, indicate that the distribution ratio of metal ions decreased with the increase of alkyl chain length attached to amidic nitrogen atom. The extraction trend observed in the present study is also in good agreement with the trend reported by others.
The separation factor of Eu(III) over Am(III) in various DGA systems are shown in Table 1. It is observed that the separation factor decreases with increase in concentration of nitric acid. Above 2 M nitric acid, the separation factor of ~1 is obtained in all cases, indicating that the extraction of Eu(III) and Am(III) is comparable under these conditions by these extractants.
Extraction stoichiometry
Figure 5 shows the variation in the distribution ratio of Am(III) as a function of DGA concentration in organic phase. It is observed that the distribution ratio increases with increase in the concentration of DGA. Usually the magnitude of slope obtained from such plot (Fig. 5) is regarded as the stoichiometry of metal-solvate complex in organic phase. It is interesting to note that the magnitude of slope varies in the order D3DODGA (3) = D2DODGA (3) < DHDODGA (4). Therefore, the number of DGA molecules associated with Am(III) increases with decreasing the chain length attached to DGA. It is quite likely that the DGA with higher alkyl chain length could be hindering the extraction and thus lowers the slope value. Due to the differences in slope, the straight line obtained for DHDODGA intersects the straight line of other DGAs at two points as shown in Fig. 5. This indicates that the distribution ratio of Am(III) in DHDODGA would be higher than that observed for both D2DODGA and D3DODGA when the concentration of DGA is more than 0.1 M. On the other hand, when the concentration of DGA is lower than 0.005 M the distribution ratio of Am(III) in DHDODGA would be less than that observed for both D2DODGA and D3DODGA. When the concentration DGA is in between 0.005 and 0.1 M the distribution ratio of Am(III) in DHDODGA would be intermediate between D2DODGA and D3DODGA systems. To confirm this, the distribution ratio of Am(III) in 0.03 M, and 0.1 M DGA/n-dodecane was measured, and the results are shown in Table 2. It is observed that the distribution ratio of Am(III) increases in the order D3DODGA < D2DODGA < DHDODGA when the concentration of DGA is 0.1 M. The order changes to D3DODGA < DHDODGA < D2DODGA when the concentration of DGA is 0.03 M. The distribution ratios of Am(III) determined using 0.005 M were not adequate (10−4) for comparing with other DGAs (Fig. 3). The study, thus, shows that depending upon the concentration of DGA employed for extraction, the order of selectivity is different.
Third-phase formation
Sasaki et al. [13] studied the third-phase formation of neodymium in symmetrical DGAs. The LOC of neodymium(III) in symmetrical DGAs increased with increase in the chain length of alkyl group attached to amidic nitrogen atom. Table 3 shows the LOC of nitric acid in various 0.1 M DGAs/n-dodecane at 298 K. It is observed that the LOC of nitric acid increases with increase in the chain length of alkyl group attached to amidic nitrogen atom in the order DHDODGA < TODGA < D2DODGA < D3DODGA. At LOC, the stoichiometry of DGA:HNO3 also increases in the same order DHDODGA < TODGA (1:1) < D2DODGA (1:2) < D3DODGA (1:3). Higher stoichiometry observed in D2DODGA and D3DODGA indicates that both the carbonyl groups present in DGA are likely to be protonated with the increase in alkyl chain length.
Among the various symmetrical DGAs, TODGA being regarded as a promising candidate for the separation of trivalent actinides from nitric acid medium. However, 0.1 M TODGA/n-dodecane forms third-phase with 6 M nitric acid and with a solution of 8 mM neodymium in 3 M HNO3 at 298 K [13]. Therefore, the modifiers such as TBP or DHOA have been used by several authors to increase the LOC of neodymium. About 0.5 M DHOA and TBP have been used by several authors to avoid third-phase formation [7–10]. The presence of high concentration of modifiers poses problems during stripping of loaded metal ion, due to the extraction of acid and other metal ions by the modifiers. In view of this, it is desirable to use solvent system that does not require any modifier. The present study (Table 3) indeed shows that the CAC of nitric acid can be increased to higher concentration levels, by simply increasing chain length of alkyl group attached to the amidic nitrogen atom. The third-phase is observed only when the initial concentration of nitric acid is increased above 3.6, 6, 7, and 12 M in DHDODGA, TODGA, D2DODGA and D3DODGA systems respectively. Since the concentration of nitric acid in HLLW varies from 3 to 4 M, the present study shows that D2DODGA and D3DODGA are superior candidates for the separation of minor actinides from nitric acid medium.
Table 4 shows the third-phase formation of neodymium(III) at various nitric acid concentrations. In DHDODGA/n-dodecane, the third-phase is formed even with small concentrations of neodymium(III) (<1 mM) in 3 M nitric acid at 298 K. The LOC of neodymium(III) in other systems decrease with increase in the concentration of nitric acid. Moreover, the LOC increases with increase in the chain length of alkyl group attached to amidic nitrogen. The CAC of neodymium(III) in 0.1 M DHDODGA/n-dodecane, TODGA/n-dodecane, and D2DODGA/n-dodecane was negligible (below the detection level by complexometric titrations) for its loading from 3 to 5 M HNO3 medium. However, it is interesting to observe from Table 4 that the third-phase in 0.1 M D3DODGA/n-dodecane occurs only when the initial concentration of neodymium reaches 105 mM in 5 M HNO3. The LOC and CAC of neodymium(III) are 24 and 69.5 mM respectively at 5 M HNO3. The stoichiometry of Nd:DGA observed at this LOC corresponds to ~1:4. When the concentration of nitric acid is lower than 5 M, the third-phase is not observed even at the initial concentration of neodymium increased to 600 mM. Under these conditions, the neodymium(III) concentration in organic phase is ~31 mM, which corresponds to the Nd:DGA stoichiometry is 1:3. However, at high concentrations of neodymium (>600 mM) in 3–4 M HNO3 medium, 0.1 M D3DODGA/n-dodecane results in the formation of third-phase with the LOC of ~32 mM. The study thus indicates that neodymium(III) could be loaded to near stoichiometric levels from 3 to 4 M nitric acid medium in 0.1 M D3DODGA/n-dodecane without the formation of third-phase.
Strontium extraction
The DGAs exhibits strong affinity towards strontium and extract significant quantities of 90Sr along with trivalent actinides. Our previous work on the unsymmetrical diglycolamide, DEHDODGA, showed that the separation factor of Am(III) over Sr(II) achieved with the use of DEHDODGA was higher than the corresponding symmetrical DGAs, TODGA and TEHDGA [14]. In this context, the distribution ratio of Sr(II) in these new unsymmetrical DGAs was also measured and the results are shown in Fig. 6. It is observed that the distribution ratio of Sr(II) increases with increase in the concentration of nitric acid and reaches a maximum at 4 M followed by decrease in D Sr(II) values. The distribution ratio decreases in the order TODGA > D2DODGA > D3DODGA. The distribution ratio of Sr(II) obtained in D3DODGA is 3–4 times lower than that observed in TODGA at 4 M nitric acid. Therefore, the extractant D3DODGA shows the advantage of poor extraction of strontium in addition to high LOCs of neodymium and nitric acid. It is interesting to observe that the distribution ratio of strontium in DHDODGA/n-dodecane is much lower than all the other DGAs. The reason for such low distribution ratio of Sr(II) in this case is not clear. However, the DHDODGA is not useful as it forms third-phase with 4 M nitric acid. Table 5 shows the comparison in the separation factor (D Am(III)/D Sr(II)) of americium over strontium in various DGAs as a function of nitric acid concentration. It is observed that the separation factor increases with the increase in the concentration of nitric acid in D2DODGA and D3DODGA systems. A separation factor as high as ~200 is obtained from 3 to 4 M nitric acid medium in 0.1 M D3DODGA system, which is indeed desirable for the separation of minor actinides from other fission products.
Conclusions
The extraction behaviour of Am(III), Eu(III), and Sr(II) in some new unsymmetrical DGAs in n-dodecane was studied as a function of various parameters. The unsymmetrical DGAs contain the octyl moiety in one arm and the alkyl group at the other arm was varied from hexyl to dodecyl moiety. They were synthesized to derive the advantage of high distribution ratio and enhanced LOC of neodymium and nitric acid. The extraction of trivalents (Am(III) and Eu(III)) in unsymmetrical DGAs decreased in the order DHDODGA > D2DODGA > D3DODGA. However, the LOC of nitric acid and neodymium (III) increased in the order of DHDODGA < TODGA < D2DODGA < D3DODGA. The distribution ratio of trivalents in TODGA was comparable with DHDODGA. Among the unsymmetrical DGAs, D3DODGA offers several advantages such as (1) high distribution ratio of trivalent lanthanides and actinides from nitric acid medium (>300 at 3–8 M HNO3) (2) low distribution ratio of strontium and thus high separation factor of americium over strontium (3) high LOC and CAC of nitric acid (4) no third-phase formation with neodymium(III) even at the initial concentration of 600 mM from 3 to 4 M nitric acid medium at 298 K. Therefore, the solvent 0.1 M D3DODGA/n-dodecane did not require any phase modifier. In view of these, the unsymmetrical diglycolamide D3DODGA is a superior candidate for the separation of trivalent actinides from HLLW, which contain ~50 mM trivalents in 3–4 M HNO3 medium.
References
Sasaki Y, Sugo Y, Suzuki S, Tachimori S (2001) The novel extractants, the diglycolamides, for the extraction of lanthanides and actinides in HNO3-n-dodecane system. Solvent Extr Ion Exch 19:91–103
Gujar RB, Ansari SA, Murali MS, Mohapatra PK, Manchanda VK (2010) Comparative evaluation of two substituted diglycolamide extractants for actinide partitioning. J Radioanal Nucl Chem 284:377–385
Narita H, Yaita T, Tachimori S (2001) In: Valiente M, Hidalgo M, Cox M (ed) Extraction behaviour of trivalent lanthanides with amides and EXAFS study on their complexes. Proceedings of the solvent extraction for the 21st century (ISEC’99). Barcelona, p 693
Ansari SA, Pathak PN, Manchanda VK, Hussain M, Prasad AK, Parmar VS (2005) N,N,N′,N′-tetraoctyldiglycolamide (TODGA): a promising extractant for actinide-partitioning from high-level waste (HLW). Solvent Extr Ion Exch 23:463–479
Mowafy EA, Aly HF (2007) Synthesis of some N,N,N′,N′-tetraalkyl-3-oxa-pentane-1,5-diamide and their applications in solvent extraction. Solvent Extr Ion Exch 25:205–224
Modolo G, Asp H, Vijgen H, Malmbeck R, Magnusson D, Sorel C (2008) Demonstration of a TODGA based continuous counter current extraction process for the partitioning of actinides from a simulated PUREX raffinate, part-II: centrifugal contactor runs. Solvent Extr Ion Exch 26:62–76
Magnusson D, Christiansen B, Glatz JP, Malmeck R, Modolo G, Purroy DS, Sorel C (2009) Demonstration of a TODGA based extraction process for the partitioning of minor actinides from a PUREX raffinate. Solvent Extr Ion Exch 27:26–35
Gujar RB, Ansari SA, Mohapatra PK, Manchanda VK (2010) Development of T2EHDGA based process for actinide partitioning. Part I: batch studies for process optimization. Solvent Extr Ion Exch 28:350–366
Deepika P, Sabharwal KN, Srinivasan TG, Vasudeva Rao PR (2010) Studies on the use of N,N,N′N′-tetra(2-ethylhexyl) diglycolamide (TEHDGA) for actinide partitioning I: investigation on third-phase formation and extraction behavior. Solvent Extr Ion Exch 28:184–201
Tachimori S, Sasaki Y, Suzuki S (2002) Modification of TODGA-n-dodecane solvent with a monoamide for high loading of lanthanides(III) and actinides(III). Solvent Extr Ion Exch 20:687–699
Vasudeva Rao PR, Kolarik Z (1996) A review of third phase formation in extraction of actinides by neutral organophosphorus extractants. Solvent Extr Ion Exch 14:955–993
Suresh A, Srinivasan TG, Vasudeva Rao PR (2009) Parameters influencing third phase formation in the extraction of Th(NO3)4 by some trialkyl phosphates. Solvent Extr Ion Exch 27:132–158
Sasaki Y, Sugo Y, Suzuki S, Kimura T (2005) A method for the determination of extraction capacity and its application to N,N,N′,N′-tetraalkylderivatives of diglycolamide-monamide/n-dodecane media. Anal Chim Acta 543:31–37
Ravi J, Suneesh AS, Prathibha T, Venkatesan KA, Antony MP, Srinivasan TG, Vasudeva Rao PR (2011) Extraction behaviour of some actinides and fission products from nitric acid medium by a new unsymmetrical diglycolamide. Solvent Extr Ion Exch 29:86–105
Sasaki Y, Choppin GR (1996) Solvent extraction of Eu, Th, U, Np and Am with N,N′-dimethyl-N,N′ di-hexyl-3-oxapentanediamide and its analogous compounds. Anal Sci 12:225–230
Suresh A, Srinivasan TG, Vasudeva Rao PR (1994) Extraction of U(VI), Pu(IV) and Th(IV) by some trialkyl phosphates. Solvent Extr Ion Exch 12:727–744
Ravi J, Prathibha T, Venkatesan KA, Antony MP, Srinivasan TG, Vasudeva Rao PR (2012) Third phase formation of neodymium(III) and nitric acid in unsymmetrical N,N-di-2-ethylhexyl-N′,N′-dioctyl-diglycolamide. Sep Purif Technol 85:96–100
Arisaka M, Kimura T (2011) Thermodynamic and spectroscopic studies on Am(III) and Eu(III) in the extraction system of N,N,N′,N′-tetraoctyl-3-oxapentane-1,5-diamide in n-dodecane/nitric acid. Solvent Extr Ion Exch 29:72–85
Deepika P, Sabharwal KN, Srinivasan TG, Vasudeva Rao PR (2010) Study on the extraction of nitric acid by N,N,N′,N′-tetra(2-ethylhexyl) diglycolamide (TEHDGA). In: Pathak PN, Sawant RM, Ramakumar KL, Manchanda VK (eds) Proceedings of DAE-BRNS biennial symposium on emerging trends in separation science and technology (SESTEC 2010). Kalpakkam, p 391
Tachimori S, Suzuki S, Sasaki Y, Apichaibukol A (2003) Solvent extraction of alkaline earth metal ions by diglycolic amides from nitric acid solutions. Solvent Extr Ion Exch 21:707–715
Acknowledgments
The authors thank Sophisticated Analytical Instrument Facility (SAIF), IIT Madras for 1H-NMR and 13C-NMR spectral recordings, Dr. K. N. Sabharwal for IR spectra recording and Ms O. K. Prajina, Cochin University for her assistance in some experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ravi, J., Venkatesan, K.A., Antony, M.P. et al. Tuning the diglycolamides for modifier-free minor actinide partitioning. J Radioanal Nucl Chem 295, 1283–1292 (2013). https://doi.org/10.1007/s10967-012-1905-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-1905-9