Abstract
Methodology for the determination of 89,90Sr, Am and Pu isotopes in complex samples is given. Methodology is based on simultaneous isolation of Sr, Y and actinides from samples by mixed solvent anion exchange chromatography, mutual separation of 89,90Sr and 90Y from actinides, mutual separation of Th, Pu and Am by extraction chromatography, quantitative determination of 89,90Sr by Cherenkov counting and quantitative determination of Pu and Am isotopes in soil and vegetation samples by alpha spectrometry. It is shown that Y and Sr can be efficiently separated from alkaline, alkaline earth and transition elements as well as from lanthanides and actinides on the column filed by strong base anion exchanger in nitrate form and 0.25 M HNO3 in mixture of ethanol and methanol as eluent. It is also shown that Pu, Am and Th strongly binds on the mentioned column, can be separated from number of elements and easily be eluted from column by water. After elution actinides were mutually separated on TRU column and electrodeposited on stainless steel disc. Examination of conditions of electrodeposition was shown that chloride-oxalate electrolyte with addition of DTPA in presence of sodium hydrogen sulphate in cell with cooling and rotating platinum anode enables deposition of actinides within 1 h by 0.8 A cm−2 current density. Obtained peaks FWHM for Pu, Am and Th isotopes are between 27 and 40 keV. Scanning electron microscopy picture and ED XRF analysis of electroplated discs showed that actinide deposition is followed by iron oxide formation on disc surface. The methodology was tested by determination of 89,90Sr, Am and Pu isotopes in ERA proficiency testing samples (low level activity samples). Obtained results shows that 89,90Sr, 241Am and 238,239Pu can be simultaneously separated on anion exchange column, 89,90Sr can be determined by Cherenkov counting with a satisfactory accuracy and limit of determination within 1–3 days after separation. 241Am and 238,239Pu can easily be separated on TRU column and determined after electrodeposition with acceptable accuracy within 1 day.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Man made radionuclides are released into environment as result of nuclear weapon testing and nuclear power plant accidents. Among many different artificial radionuclides, strontium, americium and plutonium isotopes deserve special attention. These radionuclides are important long-term pollutants with long persistence in the environment due to long half life. Differing from the analysis of gamma emitters, determination of 89,90Sr, 238,239,240Pu and 241Am requires their separation from sample. Due to their low concentration in sample and complex matrix as a result of sample decomposition, an analytical method with effective matrix separation and their mutual separation prior detection and quantitative determination is highly desired. As a rule, the determination of pure alpha and beta emitters is complicated and time-consuming and involves their chemical separation from other elements by ion exchange, extraction, precipitation or in combination of these techniques and subsequent detection on available instruments. Therefore a number of methods have been developed for determination in various kinds of samples, because numerous solutions to the problem are possible. In the last decade, progress was made by simplifying determination methods. It was realized due to of the development of new chromatographic procedures for their separation and development of ICP-MS and low level liquid scintillation counter that enables rapid and simple detection and reliable quantification [1–14]. However, despite numerous developed procedures for different kinds of samples, procedure for determination of 89,90Sr is mainly separated from procedures for the determination of other alpha emitters. Therefore, the main purpose of this paper is the development of the procedures for isolation Sr and Pu, Am from complex samples and separation of Y and Sr from alpha emitters in one step by using mixed solvent anion exchange method. Namely in our previous papers was showed(shown) how strontium and yttrium can be separated from great amount of Na, Ca, Mg and other elements on the column filled with strong base anion exchanger in nitrate form and alcoholic solution of nitric acid [15–17]. This separation is possible due to fact that the anion exchange column does not acts as ion exchange column, already, as (ad)sorption column in which separation depends on the power of electrostatic attraction between cations and nitrate ions in the solution and the functional group of exchangers. By changing of nitric acid solution polarity selective sorption of cations and their mutual separation (with alcohol addition) can be achieved [15–17]. Therefore it will be shown how this type of cation-solution-exchanger interaction can be used for the isolation of Sr, Y, Pu, Am and other alpha emitters from complex samples and mutual separation of Sr and Y from Pu and Am in one step. At the base of obtained results, method for the rapid determination of 89,90Sr by Cherenkov counting [17] and determination of Pu, Am after electrodeposition [18, 19] by alpha spectrometry is established.
It is well known that preparation of sample source for alpha spectrometry by electrodeposition has advantage over other methods due to simplicity, better homogeneity and resolution. In addition for the determination of alpha emitting radionuclides by alpha spectrometry preparation of homogenous thin layer source has crucial role in whole procedure because nuclide spectra overlapping mainly depend on inhomogenity and self absorption process. It is especially important in determination of nuclides with close alpha energies where resolution should be as high as possible. However, obtaining of a uniform source, which would provide good resolution of alpha emitters on semiconductor detector, depends on working conditions during the electrodeposition process. In last decade several procedures for source preparation by electrodeposition which are based more or less on Hallstadius and Talvitea’s [18, 19] procedures were developed [20–27]. In these procedures working conditions of the electrodeposition (composition of an electrolyte, current density, shape and distance between an anode and a cathode) have been varied in with a goal to obtain uniform distribution of nuclides and resolution as high as possible [20–27]. Therefore in this paper, influence of current density and electrolyte composition on electrodeposition efficiency and resolution are examined with emphasis on quality of alpha sources (explored by scanning electron microscopy).
Experimental
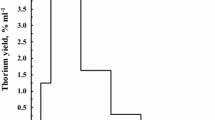
Method for simultaneous separation of Sr, Y and alpha emitters which enables their determination is based on the fact that strontium and yttrium binds on the strong base anion exchanger in NO3 − form, from alcoholic solution of nitric acid, stronger than , Ca and U and more weakly than Ra, Ba, Pb, Pu, Am and Th [15–17]—Fig. 1.
Sample preparation and Sr, Y separation
Soil sample preparation (ERA)
A 10 g soil sample was put in a 250 mL PTFE vial by adding of 1 mL of Sr and Y carrier (10 mg mL−1 each), 243Am (1 Bq), 242Pu (1 Bq) and 150 mL of deionised water and intensively stirred about 1 h. In this suspension 10 g of the cation exchanger IR-120 in H+ form (size > 250 μm) was added and stirred about 2 h by introducing a nitrogen stream (to avoid magnetic stirrer—may cause exchanger grinding). The exchanger was separated from soil on a 250 μm sieve by washing under a stream of deionised water and put into a glass column. Bound cations were eluted with 200 mL 5 M HNO3, volume had been reduced by heating almost to dryness and mixed with 100 mL of 0.25 M HNO3 in ethanol-methanol solution (1:2).
Vegetation sample preparation (ERA)
Sample was reduced to ash (burned) at 600 °C. Three grams of ash was taken for analysis, tracers 243Am, 242Pu and 1 mL of Sr and Y carrier (10 mg mL−1 each) were added, dissolved with 50 mL of 5 M HNO3 by heating under an IR lamp and filtered through G-4 sinter funnel. The solution was evaporated almost to dryness and mixed with 100 mL of 0.25 M HNO3 in ethanol-methanol solution (1:2).
Isolation from soil and vegetable samples on anion exchange column
Sample solution was passed through a column (i.d. = 1.2 cm) filled with 10 g of Amberlite CG-400 or Dowex 1 × 8 anion exchanger (100–200 mesh) in the nitrate form (bed length 15 cm). By additional passing of 100 mL solution of 0.25 M HNO3 in ethanol-methanol mixture (1:2), some alkaline, alkaline-earth and transition elements (see Figs. 1, 3; Tables 1, 2) were separated. Eluting of Y and Sr and separation from Ra, Pu, Th, Am and other elements was followed by passing of 200 mL of 0.25 M HNO3 in methanol. Fraction volume was reduced (by evaporation on hot plate at 50 °C during the elution) and Y, Sr separation procedure was followed. Th, Pu, Am, Ra and other elements were eluted with 50 mL of deionised water. Fraction volume was reduced by evaporation almost to dryness and 10 mL of 2 M HNO3 was added. Pu and Am isotopes were separated from other isotopes on TRU column [9]. TRU column (i.d. = 0.9 cm, bed height 10 cm, 2 g TRU resin) was rinsed with 50 mL 2 M HNO3–0.1 M NaNO2. NaNO2 was used to oxidize eventually presented Pu(III) to Pu(IV). After converting the column to the chloride system by means of 9 M HCl Am was striped with 40 mL 4 M HCl and Pu with 50 mL of 0.1 M ammonium oxalate. Approximate loading and stripping rate was 1 mL min−1. Alternatively Am and Pu can be separated on Dowex 1 × 8 anion exchange column in nitrate form [9].
Strontium and yttrium separation and determination
In Sr, Y fraction yttrium was precipitated with NH4OH and separated from strontium by centrifugation (separation time should be noted). Y-hydroxide was dissolved with few drops of 1 M HNO3 and precipitated again. Liquid fraction was added in Sr fraction from previous step. Y-hydroxide was washed with water, separated from solution by centrifugation, dissolved with 1 M HNO3 and transferred in PE vial. Volume should be exactly 15 mL. For the recovery determination 0.1 mL solution from PE vial was taken (from 15 mL) and transferred into 100 mL volumetric flask. Strontium was precipitated as strontium carbonate by saturated solution of ammonium carbonate and centrifuged. Precipitate was dissolved by 1 M HNO3 and Sr was precipitated again. This step should be repeated twice because strontium dissolution with 1 M HNO3 should result with transparent solution. Strontium solution was transferred in PE vial. Volume should be exactly 15 mL. For the recovery determination 0.1 mL solution from PE vial was taken (from 15 mL) and transferred in 100 mL volumetric flask. Recovery of Sr and Y was determined by atomic absorption spectrometry.
A rapid quantitative determination of 89,90Sr by Cherenkov counting [17] is based on the following 90Y activity build up and 89Sr activity decay by successive counting at different time interval and prompt counting of 89,90Sr and 90Y immediately after the 90Y separation [17]. For the activity determination by successive counting following relation was used—Method I:
Method II—90Sr–90Y equilibrium is attained in sample:
where A T1 is activity in time t 1 (cps), A T2 is activity in time t 2 (cps), A \( {}^{ 8 9}{\text{Sr}} \) is activity of 89Sr (cps), A \( {}^{ 90}{\text{Y}} \) is activity of 90Sr/90Y (cps), A c is activity concentration (Bq kg−1), A B is background activity (cps), ε1 is counting efficiency of 89Sr, ε2 is counting efficiency of 90Y, f 11 = \( e^{{ - \lambda t_{1} }} \) is decay correction of 89Sr in t 1 (= 1), f 12 = \( e^{{ - \lambda t_{2} }} \) is decay correction of 89Sr in t 2, f 21 is decay correction of 90Y in counting time t c, f 22 is decay correction of 90Y in counting time t c, λ is decay constant, t c is counting time, w 1 is fraction of equilibrium—yttrium build up in time (separtion from Sr to t 1), w 2 is fraction of equilibrium—yttrium build up in time (separtion from Sr to t 2), \( w = 1 - e^{{ - \lambda_{Y} t}} \), R is recovery, Q is sample quantity (kg or L), L d is limit of detection (counts), MDA is minimum detectable activity (Bq kg−1)
The sample preparation for alpha counting
A fraction with alpha emitters Pu, Am was evaporated almost to dryness, 100 mg NaHSO4 was added and residue was dissolved with 1–2 mL 5 M HCl. After that 4% solution of oxalic acid, 1–2 drop of ammonim hydroxy amin hydrochloride, 0.1 mmol DTPA and ammonium chloride were added. pH was adjusted to 3 with NH4OH. 20 mL of this solution was transferred in home made electrolytic cell schematically shown in Fig. 2. It consist cylinder Teflon cell (ID 2 cm, height 11 cm, 30 mL) with cathode on the bottom in metal holder which is cooled by water. Cathode is highly polish spaniel steel disc (D = 2.5 cm) with 3.14 cm2 deposition area. Anode is perforated platinum disc (d = 1.2 cm) fixed on platinum wire which rotate about 100 rpm. Before sample electrodeposition optimal working conditions were established. Source disc was analysed by scanning electron microscope equipped with energy dispersive X-ray analyzer.
Alpha spectrometry analysis
Prepared source disc was counted in Canberra 7401 alpha spectrometry chambers with PIPS Si detector (active area 300 mm2) connected with GENIE 2000 software. For the detection efficiency determination and energy calibration 241Am was used. Sample activity concentration was determined from relation \( A = \frac{{A_{{{}^{\text{net}}}} }}{R \cdot \varepsilon \cdot I \cdot Q} \) where A net is net peak area (net count per second), R is recovery, ε is detection efficiency, I is alpha intensity and Q is sample quantity. For the determination of minimum detectable activity relation (9) was used.
Chemicals, radioactive standards and proficiency testing sample
In this work Fluka Amberlite IR-120 (16–45 mesh) Amberlite CG-400 and Dowex 1 × 8 (100–200 mesh) cation and anion exchange resins were used. Standard solutions of 89Sr and 90Sr–90Y were obtained from LEA CERCA France. Standard solutions of 241Am, 243Am and 242Pu were obtained from National Institute of Standards and Technology, USA. Proficiency testing samples were obtained from Environmental Resource Association (ERA), USA. All other chemicals used in this paper were of analytical grade.
Instruments
The instrument used for detection and quantitative determination of cations was the atomic absorption spectrometer (AAS) AAnalyst 400 Perkin Elmer. Detection of radioactive strontium and yttrium was carried out on the low level Liquid scintillation spectrometer Packard TriCarb 2770 Tr/Sl and Perkin Elmer TriCarb 3180 Tr/Sl. 241Am was determined by counting on gamma spectrometer with high purity broad Ge detector Canberra equipped with Genie 2000 software. Alpha spectrometry system Canberra 7401 with Si detector was used for the determinations of alpha emitters. Scanning electron microscope JSM 7000F Joel with energy dispersive X-ray spectroscopy was used for the exploration of source disc surface.
Results and discussion
Separation of Sr, Y from Am, Pu, Th by mixed solvent anion exchange
As mentioned above, basic purpose of this paper is to present method for the isolation of Sr, Y and alpha emitters (Pu, Am) from complex samples and separation of Sr, Y from alpha emitters in one step. It is well known that Sr and Y can be isolated from a complex samples by using an anion exchanger and alcohol solution of nitric acid. Strontium and yttrium can be bonded on a strong base anion exchanger in NO3 − form from the alcohol solution of nitric acid and can be separated from alkaline elements, Ca, Mg and many other elements on the chromatographic column. The composition of alcoholic mixture, types of cations and type of exchanger determine the binding strength, i.e., the method and possibility of separation depend on them. Sr and Y can be bound only in case when anion exchanger contains quarterly ammonium functional group in presence high portion of alcohol and nitrate ion in exchanger structure. It should be mentioned that binding strength depend on some kind of electrostatic (ad)sorption i.e. the electrostatic attraction between cations and nitrate ions in the solution and the quarterly ammonium functional group of exchangers [15–17]. The actual binding mechanism is unknown and it was previously considered [15, 16], when the method of separation of strontium from alkaline and alkaline earth element was developed. From these studies it emerged that this type of binding also allows separation of Y and Sr from alpha emitting isotopes because Pu, Am and Th more strongly binds on the exchanger than Sr and Y. Namely Table 1 shows exchanger binding ability (and distribution coefficients) for the Sr,Y, some alpha emitters and other cations. It is obvious that Th, Am, and Pu more strongly binds on the anion exchanger from 0.25 M HNO3 in methanol than Y and Sr while U alkaline and many transition elements weakly or does not bind on exchanger. A binding strength of Y and Sr increase with decreasing of alcohol polarity while binding strength of Th, Pu and Am decreases with decreasing of alcohol polarity and that there is no significant difference between binding ability of Dowex and Amberlite exchangers. In practice it means that presence of ethanol in methanol will increase binding strength of Y and Sr and decrease binding strength of Pu, Am and Th. It is important for the isolation from real sample because increasing of binding strength of Sr and Y reduces their earlier elution from the column while decreasing of binding strength of Pu, Am and Th is not so significant that disables their mutual separation and causes loss during the separation. In addition, presence of ethanol in methanol enables efficient separation from Ca and U as it is shown in Fig. 3. On the other side, increase in binding strength will result with increasing of retention times of Sr and Y and slow down whole procedure. In order to avoid prolonged Sr, Y elution time, mixture of methanol-nitric acid–water can be used.
Results in Tables 2 and 3 show that binding strength of cations decreases with increasing of eluent polarity. So presence of small amount of water in 0.25 M HNO3 in methanol causes sharp falls of their bonding ability—Table 2. Therefore elution with addition of water can cause earlier elution of alpha emitters in Sr, Y fraction and should be avoided (except last step when alpha emitters are stripped from column). The results in Table 3 shown that increasing of acid concentration in methanol also reduces their binding strength but not nearly as sharp as water. In this case difference between distribution coefficients of Y, Sr and Pu, Am is high enough that enables their mutual separation without risk of premature elution of Pu and Am. It should be mentioned that mutual separation of Th, Am and Pu as well as mutual separation of Y and Sr at this manner is not possible. In addition, Ba, Ra, Pb, lanthanides and Bi are strongly bound on anion exchanger from alcohol and can not be separated from Pu and other alpha emitters.
These results enable creating the methodology for the isolation of Sr, Y, Pu, Am and Th from different kinds of samples and mutual separation of Sr, Y from alpha emitters. Figure 1 shows the methodology for the separation and quantitative determination which is used in this paper and it is described in experimental part. As can be seen, methodology include separation of alkaline, some transition elements, Ca, Mg and U from Sr, Y and alpha emitters by using 0.25 M HNO3 in ethanol–methanol solution, elution and separation of Y, Sr from Pu, Am and other bound cations by using 0.25 M HNO3 in methanol as eluent, followed by stripping of alpha emitters and other cations with water. Pu and Am are separated on TRU (column because separation on TRU column) that enables separation from elements such as Pb, Bi, Ra, Ba and lanthanides in short time.
As it can be seen from previous results by changing of eluent composition, retention time can be changed and method can be adapted according to specific requirements. For example, if sample contains small amount of Ca, Y and Sr can be bound and eluted with 0.5 M nitric acid–methanol solution without risk of their loss or earlier Pu and Am elution. It should be mentioned that Pu, Th and Am binds from nitric acid on Dowex 1 × 8 anion exchange column and can be mutually separated by selective elution at the same column. As we have seen that there is no difference between binding and separation on Dowex and Amberlite exchangers it can be expected that after elution of Sr and Y, americium will be eluted with 8 M HNO3, Th with 9 M HCl and Pu with 9 M HCl–0.1 M NH4I. However, in this case separation is directed by classical ion exchange mechanism in which element oxidation state play important role. Pu can be in Pu(III) and Pu(IV) oxidation state and do not bind on anion column as Pu(III)-complex from nitric acid. This is in contrast with binding from alcoholic solution where Pu oxidation state does not prevent its binding. As for the separation of Pu from other elements on anion exchange column, adjustment of its oxidation state is required. As Pu oxidation states in alcoholic solution of nitric acid isn’t known exactly (most probably as Pu(IV,VI)) this type of separation wasn’t used in this study. Namely using of same column for the mutual separation of alpha emitters in case when different mechanism governs their binding requires additional examination before practical implementation and will be given in another paper.
Preparation of alpha source by electrodeposition
For the determination of alpha emitting radionuclides by alpha spectrometry preparation of homogenous thin layer source has crucial role in whole procedure because nuclide spectra overlapping mainly depend on inhomogenity and self absorption process. It is especially important in determination of nuclides with close alpha energies where resolution should be as high as possible. However, obtaining of a uniform source, which would provide good resolution of alpha emitters on semiconductor detector, depends of working conditions during the electrodeposition process. It was seen in different literature sources that described procedures are based more or less on Hallstadiusa and Talvitea’s procedures [18, 19]. Conditions of the electrodeposition (a composition of an electrolyte, a current density, a shape and a distance between an anode and a cathode) have been varied for obtaining of uniform source which will enable determination of alpha emitters with close energy. Therefore in this paper conditions of electrodeposition were examined. The goal was finding conditions at which the alpha emitters would be deposited at a cathode in short time with high efficiency.
For the electrodeposition a chloride-oxalate electrolyte with addition of ammonium salt DTPA and hydroxyl ammonium hydrochloride was chosen. DTPA as a chelating agent (for lanthanides) and with good solubility in an acid medium provides high deposition efficiency for all alpha emitters, blocks its polymerization but prolongs a time of electrodeposition—Fig. 4. Hydroxyl ammonium hydrochloride stabilises yields of deposited radionuclides, reduces and restrains plutonium in the form of Pu(III). Acidity has been changed during an electrodeposition because H+ has been reduced at a cathode (H2 evolution) and hydrogen ion has been refunded by migration, diffusion and even oxidation of water molecules. Entire process depends on the cathode current density. Most of H+ ions have been ejected at high current density (applied in this work) at the beginning of electrodeposition in strong acids and that ejecting is faster than replacement from weak acid or water oxidation. The consequence is a rapid increase of pH [21, 22, 25–27] in first several minutes of electrodeposition and then decreasing caused by compensation of H+ ions from dissociation and/or decarbonisation of oxalic acid. The optimal yield could be achieved when pH is equal to pK of the weak acid. Working in the acid medium reduces ability of precipitation and producing of insoluble hydroxide. In this context Janda et al. [25] showed that pH range 1.6–2.2 for sulphate electrolyte enable high efficiency of electrodeposition. They also observed that crystals dropping out in the stock solution by decreasing the pH-value below the value of 1.8 and on the contrary, rising the pH-value above 2.4 the interruption of electrodeposition and the inclination of degradation of the resolution [25].
The rate of the electrodeposition depends on the current density, what can be seen from the results demonstrated in Fig. 5. The results of 241Am deposition show that almost 100% yield have been reached in approximately 50 min if a current density is 0.8 A cm−2. It is well known that higher current density causes higher rate of deposition, but simultaneously the temperature of electrolyte rises and this leads to the formation of bubbles which act as insulators decreasing the active area of deposition and thereby increasing current density, which leads to higher granularity of deposit [25]. Therefore our cell is cooled and the electrolyte is agitated with a rotating anode in order to decrease the effect of the temperature and to avoid overheating. It has been observed that with current density of 0.8 A cm−2 there is no overheating and boiling of the electrolyte, but that starts at the current density of 0.96 A cm−2.
The efficiency of electrodeposition and a quality of a source are implicated by a shape of the anode and by the distance between the anode and the cathode. It is known that uniform current distribution results with the forming of the uniform layer, and that it depends on geometry of the system and applied voltage. It is eligible that the distance between the anode and the cathode (in used cell 5 mm) is as little as possible because this enables working with lower voltage and uniform current distribution. A shape of the anode significantly influences on the distribution of deposited nuclides and therefore it should be selected the shape of the anode which will give uniform distribution. As Benedik and Klemenčić showed the shapes of spire or riddle gives the best distribution and results with uniform layer of nuclides on the cathode in comparing with other tested shapes [27]. Hence a perforated anode with a shape similar to a riddle in was used in present work—Fig. 2. Using described procedure, Pu and Am were electrodeposited from model samples and ERA water samples on stainless steel cathode. Obtained spectra was analysed and compared with spectra of Analytics standard source (electrodeposited mixture 243Am and 241Am on stainless disc). Obtained results showed that resolution is 8 keV/channel and full width at half maximum is in range 27 to 40 keV. In Fig. 6 spectrum of 239Pu and 241Am obtained after direct electrodeposition from water sample ERA MRAD12 (without separation) and spectrum of standard source is shown. It is obvious that there in no significant difference between spectra of ERA water and standard sample. It should be mentioned that water sample also contains 238Pu so that obtained 241Am peak is really peak of both isotopes.
However, it shown that in general the quality of electrodeposits sources improves with decrease of deposition current which is attributed to the formation of smaller aggregates of hydroxides under low current densities [26, 28]. Hansen theory of electrodeposition implies that the deposition yield increases with decrease of the current density since the lower so called hydroxyl layer, the easier diffusion of actinide hydrolyzed species to the surface of cathode through hydroxyl layer [22, 29, 30]. Beesley et al. [26, 31] also showed that alpha source contains Pt atoms which evolve with electrodeposition time, to some Pt aggregates which can contribute to deteriorate the energy resolution of the alpha spectra obtained from source even in deposition of nuclide which contributes with significant mass to the deposit thickness. Therefore source discs were analysed before and after the electrodeposition by Scanning electron microscope to have more detailed access to the structure of the obtained layer. Another analysis of an electrodeposited layer was taken by the ED XRF. The electrodeposition was performed in previously described way with addition of HF to prevent Pt dissolution and aggregation on disc surface. All analysed sources gave correct alpha spectra. The SEM pictures of the analysed source discs are shown in Figs. 7, 8, and 9 and the results of the ED XRF analysis are shown in the Table 4. Figure 7 shows SEM picture of disc surface before electrodeposition and Fig. 8 pictures of disc surface after electrodeposition at different place. The results in the Table 4 indicate that during a electrodeposition, an oxide layer of iron with different composition was formed. This denotes that deposition of actinides is associated with forming of previously mentioned layer. In fact, looking at the growth of oxygen’s proportion on the analysed surface and observing the frames it can be assumed that the main part of the surface is an iron (hydro)oxide and that oxides of the actinides have been formed together with forming of iron oxide. It can be also seen that Pt aggregates were found on the surface of oxide layers. Therefore, it can be assumed that a quality of source depends on a forming of the iron oxide layer and of the allocation of radionuclides in the layer [26, 31]. Figure 10 shows impact of iron concentration in a solution on efficiency of electrodeposition of Am and Ra. The iron does not affect on the efficiency of electrodeposition if its concentration in the solution is lower then 5 mg L−1. In addition, it can be assumed that low concentration of iron ions in the solution do not influence on oxide layer because it was not noticed a distortion of alpha spectra. It should be mentioned that Th and other naturally occurred alpha emitters can be electrodeposits and determined. As it is shown that Ra, Pb and Th are strongly binds on anion exchanger it is possible to isolate and deposits them onto disc and determine by alpha spectrometry.
SEM picture of disc surface before electrodeposition (Table 4 XRF analysis of surface)
Results of determination of 89,90Sr
The 89,90Sr, 238,239Pu and 241Am were determined in ERA proficiency testing samples of vegetation and soil. The samples had not contained 89Sr so that in some samples known activities of 89Sr were added. 89,90Sr were isolated and separated from alpha emitters on anion exchanging column and determined on the LSC as previously described. 89,90Sr was determined at two way; by successive Cherenkov counting through 90Y build up and 89Sr decay according to Eqs. (1–6) as well as by Cherenkov counting of 90Y where 90Sr was determined through 90Y and 89Sr was determined from difference between gross activities, AT, (counted immediately after separation) and 90Y (Eqs. 7, 8). Obtained results are shown in Table 5. Results show that discrepancies between obtained and assigned values for 89;90Sr are in range of ±18 %. Discrepancy depends on recovery, level of activity of 89,90Sr, their activity ratio and other parameters and it was discussed in detail in our previous paper as well as limit of detection and determination [17].
Results of determination 238,239Pu and 241Am
238,239Pu and 241Am are isolated from ERA soil and vegetation sample by mixed solvent anion exchange mutually separated on the TRU column (due to 238Pu, 241Am energy overlapping), electrodeposits and quantitatively determined by alpha spectrometry as described in experimental part. Activity of 241Am is also determined by gamma spectrometry. Obtained results are shown in Table 6. From these results it can be seen that deviation between obtained and assigned results amounts maximally 21% for determination of 238Pu. This discrepancy corresponds with decreasing of recovery of Pu and Am in soil samples. It should be mentioned that recovery depends on sample preparation, separation step and electrodeposition. As isolation procedure contains binding of radionuclide on cation exchanger and separation on anion exchange column some loss of radionuclide can be expected, but in which extent in each phase is hard to determine. Pu and Am are strongly bound on anion exchanger and greater loss in separation step is not expected but in phase of binding on cation exchanger can be, because cation exchanger has limited capacity and binds only surface adsorbed and acid soluble fraction of cations from a (soil) samples. Therefore 241Am was measured by gamma spectrometry in soil samples before and after treatment with cation exchanger and on cation exchanger after the elution with 5 M HNO3. These results shown that 241Am was present in small amount (below 2 Bq kg−1) in soil samples after treatment but on cation exchanger after elution with 5 M HNO3 was not detected, so it can be concluded that despite sufficient quantity of a exchanger [17] certain loss can always be expected in this stage. It should be mentioned that cation exchanger can bind surface adsorbed and acid soluble fraction of cations from a soil samples. Namely cation exchanger in H+ forms acts as acid and can dissolve carbonates and some other compounds but it has limited binding ability from hardly soluble compounds. This fact restricts its use in analysis of samples where it is expected that isotopes are in insoluble form because its uses can lead to wrong result. Namely exchanger will bind tracer but not bind all Pu and Am (only available) so that too high results for recovery can be expected. In that case best solution is total dissolving of sample (by microwave digestion).
Conclusion
The obtained results have shown that at Y and Sr can be efficiently separated from alkaline, alkaline earth and transition elements as well as from lanthanides and actinides on the column filled by strong base anion exchanger in nitrate form and 0.25 M HNO3 in mixture of ethanol and methanol as eluent. This separation in combination with Cherenkov counting enables rapid determination of 89,90Sr with acceptable accuracy. It is also shown that Pu and Am strongly binds on the mentioned column, can be separated from number of elements, eluted from column by water, mutually separated on TRU column and electrodeposited on stainless steel disc. Electrodeposition from chloride-oxalate electrolyte with addition of DTPA in presence of sodium hydrogen sulphate in cell with cooling and rotating platinum anode enables deposition of actinides within 1 h by 0.8 A cm−2 current density. At this manner sample source which gives good resolution in alpha spectrometric measurement can be prepared. The results obtained by alpha spectrometry showed that the peak width at half maximum range of 27–40 keV and allows determination of 241Am and 238,239Pu with acceptable accuracy. Results obtained by scanning electron microscopy and ED XRF analysis of electroplated discs showed that actinide deposition is followed by iron oxide formation on disc surface as well as platinum aggregation on oxide surface.
References
St-Amant N, White JC, Rousseau ME, Lariviere D, Ungar RK, Johnson S (2011) Appl Radiat Isot 69:8
Addleman RS, O’Hara MJ, Marks T, Grate JW, Egorov OB (2005) J Radioanal Nucl Chem 263(2):295
Jassin IE (2005) J Radioanal Nucl Chem 263(1):95
Dulanská S, Remenec B, Mátel L, Galanda D (2011) J Radioanal Nucl Chem 287(3):841
Eikenberg J, Jäggi M, Beer H, Rüthi M, Zumsteg I (2009) Appl Radiat Isot 67:776
Lee MH, Ahn HJ, Park JH, Park YJ, Song K (2011) Appl Radiat Isot 69:295
Maxwell SL, Culligan BK, Noyes GW (2011) Appl Radiat Isot 69:917
Harrison JJ, Zawadzicki A, Chisari R, Wong HKY (2010) J Environ Radioact 87:1
Jakopič R, Tavčar P, Benedik L (2007) Appl Radiat Isot 65:504
Dulanská S, Remenec B, Mátel L, Antalı I (2011) J Radioanal Nucl Chem. doi:10.1007/s10967-011-1370-x
Dauner J, Workman S (2012) J Radioanal Nucl Chem. doi:10.1007/s10967-012-1676-3
Sengupta A, Adya VC, Godbole SV (2011) J Radioanal Nucl Chem. doi:10.1007/s10967-011-15896-6
Kuruc J, Harvan D, Galanda D, Mátel L, Jerigová M, Velič D (2011) J Radioanal Nucl Chem 289(2):611
Macsik Z, Shinonaga T (2010) Appl Radiat Isot 68:2147
Grahek Ž, Eškinja I, Košutić K, Lulić S, Kvastek K (1999) Anal Chim Acta 379:107
Rožmarić M, Gojmerac Ivšić A, Grahek Ž (2009) Talanta 80(1):352
Grahek Ž, Karanović G, Nodilo M (2011) J Radioanal Nucl Chem. doi:10.1007/s10967-011-1441-z
Talvitie NL (1972) Anal Chem 44:280
Hallstadius L (1984) Nucl Instrum Meth Phys Res 233:266
Bajo S, Eikenberg J (1999) J Radioanal Nucl Chem 242(3):745
Lee MH, Lee CW (2000) Nuclear. Instrum Methods Phys Res A 44(7):593
Tsoupko-Sitnikov V, Dayras F, De Sanoit J, Filossofov D (2000) Appl Radiat Isot 52:357
Salar Amoli H, Barker J, Flowers A (2006) J Radioanal Nucl Chem 268(3):497
Plions A, Haas D, Landsberger S, Brooks G (2008) J Radioanal Nucl Chem 276(3):369
Janda J, Sladek P, Sas D (2010) J Radioanal Nucl Chem 286(3):687
Beesely AM, Crespo MT, Weiher N, Tsapatsaris N, Cozar JS, Esparza H, Mendes CG, Hill P, Schroeder SLM, Montero-Cabrera ME (2009) Appl Radiat Isot 67:1559
Klemenčić H, Benedik L (2010) Appl Radiat Isot 68:1247
Currie L (1968) Anal Chem 40:586
Hansen PG (1959) J Inorg Nucl Chem 12:30
Hansen PG (1960) J Inorg Nucl Chem 17:232
Martin Sanchez A, Nuevo Sanchez MJ, Rubio Montero MP, Mendez Vilas A (2002) Appl Radiat Isot 56:31
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grahek, Ž., Nodilo, M. Continuous separation of Sr, Y and some actinides by mixed solvent anion exchange and determination of 89,90Sr, 238,239Pu and 241Am in soil and vegetation samples. J Radioanal Nucl Chem 293, 815–827 (2012). https://doi.org/10.1007/s10967-012-1740-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-1740-z