Abstract
A comparison of sequential extraction methods proposed by F.I. Pavlotskaya [1, 2] and A. Tessier [3] for fractionation of technogenic (137Cs and 90Sr) and natural (226Ra, 232Th, and 238U) radionuclides from soils was performed. It is shown that both methods provide comparable results in the extraction of various forms of occurrence of technogenic radionuclides. Furthermore, both methods indicate a significantly higher availability of 90Sr to plants and its greater ability to migrate with downward soil solution flows in comparison with 137Cs. However, when used for the assessment of the occurrence forms of natural heavy radionuclides, the two methods provide inconsistent results. The Tessier sequential extraction method indicates higher contents of compounds available to plants and mobile compounds in comparison with the Pavlotskaya method. A possible reason behind this may be the soil chemistry complexity of radionuclides such as 232Th and 238U that feature polyvalence and a strong tendency for hydrolysis and complex formation; in addition, their behavior may be affected by various carriers. These elements form a broad range of compounds that change one into another with changes in the chemical conditions; this complicates accurate comparison of the composition of their forms extracted by reagents used in the above methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The presence of various forms of occurrence of radionuclides in soils differing by their mobility, biological availability, and fixation mechanisms requires detailed study. The extraction methods used for radionuclide speciation assessments significantly affect the results of such studies.
Currently, there are plenty of methods used for fractional separation of soil radionuclides; a comparative analysis of these techniques is required to be able to compare data obtained by different methods.
The extraction methods involve sequential treatment of soil specimens with a series of chemical agents selectively dissolving groups of radionuclide compounds with different properties. Each successive agent must either be stronger (i.e., more aggressive) than the previous one or have a different nature. The methods are pretty conventional because it is difficult to select in laboratory conditions a series of reagents selectively extracting radionuclide compounds from soil with various soil components or grouped by specific properties. Most methods sequentially extract groups of radionuclide compounds with the same solubility; such groups are conditionally considered groups of soil radionuclide forms with similar properties [2, 4, 5]. The strength and nature of bonds between a radionuclide and the soil adsorption complex is often considered the most important property of various groups directly affecting radionuclide mobility in soil.
Despite the existing difficulties, sequential extraction methods are broadly used in geochemistry, soil science, and radioecology because their results make it possible to get a better understanding of the behavior of chemical elements, including radionuclides, in the environment.
The literature includes plenty of studies involving the application of various sequential heavy metal extraction methods on same specimens [6–11], reviews dedicated to this issue [4, 5], etc. The number of studies comparing sequential extraction methods used in radionuclide studies is significantly fewer.
For instance, P. Blanco et al. [12] have compared the methods proposed by A. Tessier [3] and M.K. Schultz [13] using a natural soil specimen with high activities of 226Ra, 238U, and 230Th as an example. It was shown that these two procedures provide different results and should be interpreted independently of one another for each radionuclide.
T.A. Goryachenkova et al. [2] have compared several techniques used for extraction of plutonium forms of occurrence (the Pavlotskaya [1], Miller [14], Tessier [3], and Smith [15] methods) using as an example a bottom sediment specimen collected in a water body located within the territory of the Mayak Production Association. In that case, the experiment has shown a satisfactory consistency of the data on plutonium contents in the following forms obtained using the methods compared: exchangeable, highly soluble, bonded with organic matter, and bonded with iron and manganese oxides.
The purpose of this study was to compare two methods used for sequential extraction of forms of radionuclide (137Cs, 90Sr, 226Ra, 232Th, and 238U) occurrence: the Pavlotskaya method traditional for Russian radioecology [1, 2] and the Tessier method, which is most popular in the foreign literature [3].
MATERIALS AND METHODS
The research subject was a soil specimen collected in 2001 in the podzolic horizon of a sod–low-podzolic sandy soil formed under a broad-leaved–coniferous forest (Krasnogorsk district, Bryansk oblast). In 1986, the soil contamination density with 137Cs in the study area was 2.26 MBq/m2. The main dose contributors in this area were two components of the Chernobyl fallout: 134Cs and 137Cs; the share of 90Sr in the total contamination had not exceeded several percentage points [16].
The studied specimen features a very acidic reaction, low organic carbon content, and low bases saturation degree of the soil adsorption complex (Table 1). By its granulometric composition, the specimen is unconsolidated sand. The shares of physical clay (<0.01 mm) and physical silt (<0.001 mm) constitute 3.1 and 0.5%, respectively. The specimen mostly consists of coarse, medium, and fine sand.
The studied soil specimen features high specific activities of 137Cs and 90Sr (Table 1). The activities of natural radionuclides are consistent with the levels typical for soils of European Russia [17].
For our comparative study, we have chosen the method for sequential extraction of radionuclide occurrence forms proposed by F.I. Pavlotskaya and further developed in studies by a large group of radioecologists [1, 2, 18–20] and other researchers. The method involves the extraction of five radionuclide fractions having bonds of various strength and origin with the solid phase of the soil and extraction of radionuclides from amorphous compounds by applying Tamm’s reagent to a separate quantity of soil (Table 2).
The second technique selected for the comparative study was the Tessier method [3]. It also includes the extraction of five radionuclide fractions from various soil components (Table 3).
An average sample was prepared for the experiment; 100-g quantities were used for the fractionation.
Radiochemical separation of radionuclides was performed in the obtained extracts. First of all, organic matter was destroyed in all extracts by boiling with Н2О2 under acidic conditions; then the 137Cs specific activity was measured by a Multirad gamma spectrometer (Russia) with a NaI(Tl) scintillation detector 63 × 63 mm in size. The concentrations of 232Th, 238U, and heavy metals were measured in the same extract by an Agilent ICP-MS 7500a inductively coupled mass spectrometer (United States).
Then the extract was divided into two halves. In the first half, 90Sr was separated from other radionuclides using the oxalate method [21]. The specific activity of 90Sr was measured by a Multirad beta spectrometer (Russia) with a plastic scintillation detector 70 mm in diameter after retrieving the balance between 90Sr and its daughter product, 90Y.
Radium isotopes were extracted from the second half through cosedimentation with BaSO4 after the preceding concentration of other radionuclides on FeOH3 [22]. The 226Ra activity was determined by threefold measurements of the total alpha activity of the radium preparations by a Multirad alpha spectrometer (Russia) with a ZnS(Ag) scintillation detector 70 mm in diameter 1–2, 9–10, and 28–30 days after BaSO4 sedimentation. The activity of 226Ra was calculated taking into account the 226Ra, 223Ra and 224Ra decay and accumulation of their decay products [23].
The content of each radionuclide in soil fractions was calculated in percentage points of its total content in the quantity of the soil.
RESULTS
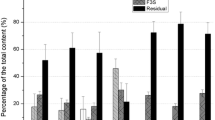
The fractionation of soil components in the studied specimen using the Pavlotskaya method 15 years after the Chernobyl accident has shown that 137Cs is mostly concentrated in the residuum remaining after the series of extractions (Table 4). This fraction contains 85.6% of the radionuclide situated in the interwrapper space of crystalline lattices of secondary clay minerals (mostly illite-structured ones) [24]. Up to 10.2% of the total 137Cs activity in the soil is attributed to the acid-soluble fraction that includes, according to [4], amorphous (partially) and crystalline Fe and Al oxides and products of interactions between humic substances on the one hand and stable sesquioxides and clay minerals on the other hand. The sum of mobile fractions is 4.2%. The exchangeable highly soluble fraction (2.2%) predominates among them; it includes both exchangeable proper and specifically sorbed forms. The mobile fraction (1.5%) takes second place; it contains the radionuclide associated with Mn oxides, readily oxidizable organic compounds, and partially with amorphous Fe and Al oxides [18]. Only 0.5% of the total 137Cs activity in the soil is attributed to the most mobile water-soluble fraction. Tamm’s reagent extracts 3.6% of the total 137Cs content with amorphous compounds.
After fractionation performed using the Tessier method, 91.0% of the total activity remains in the residuum fraction combining compounds bonded with crystalline Fe and Al oxides and 137Cs contained in secondary minerals. Here, 5.5% of 137Cs is bonded with organic matter. The sum of the most mobile radionuclide fractions amounts to 3.5%; out of that amount, 2.7% is bonded with amorphous Fe oxides and Mn oxides, while the exchangeable and carbonate fractions constitute 0.2 and 0.6% of the total 137Cs activity, respectively.
The distribution of 90Sr activities by fractions differs significantly from the 137Cs distribution. If the fractionation is performed using the Pavlotskaya method, the maximum activity share (63.3%) is attributed to the exchangeable fraction. A significantly lesser share (15.4%) is attributed to the mobile fraction, while water-soluble compounds contain 3.9% of the total activity. The sum of mobile 90Sr fractions is 82.6%. The acid-soluble fraction (15.5%) predominates among the difficult-to-extract ones, while the residuum constitutes 1.9%. Tamm’s reagent extracts 25.2% of the total 90Sr activity.
If the fractionation is performed using the Tessier method, the maximum 90Sr activity is attributed to the exchangeable (35.5%) and carbonate (30.4%) fractions. It is necessary to note that radionuclides bonded with Fe and Mn oxides partially pass into this fraction in acidic soils [3, 4]. In addition, 7.1% of the total 90Sr activity is associated with Fe and Mn oxides, 19.0% with organic matter, and 8.0% with crystalline Fe and Al oxides and crystalline lattices of minerals.
Despite the similarity of their chemical properties, the distribution of 226Ra by fractions differs significantly from the 90Sr distribution. If the fractionation is performed using the Pavlotskaya method, the sum of mobile fractions is 5.8%, and 226Ra is roughly equally distributed between the water-soluble (1.4%), exchangeable (1.9%), and mobile proper (2.5%) fractions. The maximum amount of 226Ra is bonded with Fe and Al oxides and stable organic compounds (61.3%). The residuum constitutes 32.9%. The share of 226Ra bonded with amorphous Fe and Al compounds extracted by Tamm’s reagent is 13.6%.
If fractionation is performed using the Tessier method, the maximum amount of 226Ra (73.3%) is bonded with crystalline Fe and Al oxides and crystalline lattices of minerals. The rest of the total 226Ra activity is roughly equally distributed between other fractions: exchangeable (6.5%), carbonate (5.6%), bonded with Fe and Mn oxides (7.7%), and bonded with organic matter (6.9%).
The distributions of 232Th and 238U by fractions are substantially similar to each other. If the fractionation is performed according to the Pavlotskaya method, the residuum contains the maximum amount of 232Th (80.5%), while the sum of mobile fractions is 12.7%. Speaking of 238U, the residuum contains 80.0%, while the sum of mobile fractions is slightly lower than that for 232Th: 7.3%. The differences are caused by a higher share of acid-soluble 238U compounds in comparison with 232Th. Tamm’s reagent also extracts larger amounts of 238U (23.4%) in comparison with 232Th (16.0%).
If fractionation is performed using the Tessier method, the residuum contains 76.6% of 232Th and 77.3% of 238U. The shares of exchangeable and carbonate fractions differ insignificantly. The maximum differences are observed in relation to the fraction bonded with Fe and Mn oxides (1.2% of 232Th and 6.4% of 238U) and the one bonded with organic matter (8.5% of 232Th and 2.5% of 238U).
DISCUSSION
Cesium-137
The data obtained indicate (Tables 4, 5) that the share of the exchangeable fraction extracted using the Tessier method is significantly lower than the sum of the exchangeable and water-soluble fractions extracted using the Pavlotskaya method. These differences likely originate from the preferential synthesis of exchangeable radiocesium forms on selective sorption spots located on frayed edge sites of layered clay minerals [24–27]. Such sorption spots also feature high selectivity to NH4+ ions, which determines their higher displacing power in comparison with Mg2+ ions. Apparently, the carbonate fraction extracted using the Tessier method contains, in addition to specifically sorbed ions, a portion of exchangeable ions that have not passed into the exchangeable fraction; in acidic soils, it also contains a portion of cesium bonded with amorphous iron and manganese oxides [3, 4]. The sums of mobile fractions extracted using the Pavlotskaya method (the water-soluble fraction, exchangeable fraction, and mobile proper fraction) and according to the Tessier method (the exchangeable fractions, carbonate fractions, and the fractions bonded with Fe and Mn oxides) are comparable with each other: 4.2 and 3.5% respectively. The sum of mobile fractions extracted using the Pavlotskaya method is somewhat higher because the mobile proper fraction includes a portion of cesium bonded with organic matter. The share of 137Cs bonded with amorphous iron compounds, including water-soluble compounds and a portion of exchangeable ions, extracted from a separate soil quantity using the Pavlotskaya method is comparable with the sum of mobile fractions extracted using the Tessier method: 3.6 and 3.5%, respectively. Apparently, this is due to the high displacing power of NH4+ ions contained in Tamm’s reagent with respect to monovalent 137Cs ions that are completely displaced from the soil adsorption complex. The share of the acid-soluble fraction extracted using the Pavlotskaya method is higher than the share of the fraction bonded with organic matter extracted using the Tessier method because the acid-soluble fraction includes 137Cs bonded with iron and aluminum oxides and hydroxides.
Strontium-90
The share of the exchangeable fraction extracted using the Tessier method is almost one-half as high as the sum of the exchangeable and water-soluble fractions extracted using the Pavlotskaya method. Apparently, this is because the exchangeable fraction extracted using the Pavlotskaya method includes, in addition to exchangeable ions, all specifically sorbed 90Sr ions (i.e., the most weakly bound ions contained in outer- and inner-sphere complexes fixed on defects in crystalline lattices) [5]. A comparison of the sum of the exchangeable and water-soluble fractions extracted using the Pavlotskaya method and the sum of the exchangeable and carbonate fractions extracted using the Tessier method gives pretty close values: 67.2 and 65.9%, respectively. A comparison of the sums of mobile fractions extracted using the Pavlotskaya and Tessier methods also gives consistent results: 82.6 and 73.0%, respectively. The sum of mobile fractions extracted using the Pavlotskaya method is somewhat higher because the mobile fraction includes a portion of 90Sr bonded with organic matter. The share of the acid-soluble fraction extracted using the Pavlotskaya methods is roughly consistent with the share of the fraction bonded with organic matter extracted using the Tessier method. This is likely because 90Sr is absorbed more intensely by moderately oxidizable compounds and those resistant to oxidation, not by those resistant to deoxidization.
It is important to note that both the Pavlotskaya and Tessier methods indicate a significantly larger amount of 90Sr in the sum of mobile compounds in comparison with 137Cs. In other words, both methods indicate a significantly higher availability of 90Sr to plants and its greater ability to migrate with downward soil solution flows in comparison with 137Cs.
Therefore, a comparison of the Pavlotskaya and Tessier sequential extraction methods in relation to the fractionation of technogenic radionuclides (137Cs and 90Sr) gives pretty consistent results (provided that acid-soluble 90Sr forms in the soil extracted using the Pavlotskaya methods are equivalent, at least partially, to its forms bonded with organic matter extracted by the Tessier method).
Radium-226
The share of the exchangeable fraction extracted using the Tessier method is significantly higher than the sum of the exchangeable and water-soluble fractions extracted according to the Pavlotskaya method. In this case, the higher displacing power of divalent Mg2+ ions (in comparison with monovalent \({\text{NH}}_{4}^{ + }\) ions) plays the key role in the extraction of divalent 226Ra ions from the soil adsorption complex. The sum of mobile fractions extracted using the Pavlotskaya method is almost 3.5 times lower than the sum of mobile fractions extracted according to the Tessier method. Concurrently, the share of 226Ra bonded with amorphous iron compounds, including water-soluble compounds and a portion of exchangeable ions, extracted from a separate soil quantity using the Pavlotskaya method is more comparable with the sum of mobile fractions extracted using the Tessier method: 13.6 and 19.8%, respectively. The share of the acid-soluble fraction extracted using the Pavlotskaya method is significantly higher than the share of the fraction bonded with organic matter extracted using the Tessier method because the acid-soluble fraction includes 226Ra bonded with Fe and Al oxides and hydroxides; apparently, they absorb 226Ra more intensely than organic compounds [28].
Overall, the 226Ra distribution by fractions differs significantly from the 90Sr distribution; this is probably because they enter soil from different sources. 90Sr has entered it with the aerial–technogenic fallout; accordingly, it is not bonded with crystalline lattices of minerals. 226Ra was released from parent rocks in the course of natural weathering processes and additionally accumulated from degraded minerals in poorly soluble iron and aluminum oxides [28]. Furthermore, the behavior of 90Sr in soil is mainly controlled by its isotopic carrier, stable strontium, while the behavior of 226Ra having no stable isotopic carrier may be significantly affected by typomorphic soil elements (iron, aluminum, and calcium).
Thorium-232
The sum of the exchangeable and specifically sorbed fractions extracted using the Tessier method is significantly higher than the sum of the water-soluble and exchangeable fractions extracted using the Pavlotskaya method. This may be caused by the fuller extraction of 232Th ions by divalent Mg2+ ions in comparison with monovalent \({\text{NH}}_{4}^{ + }\) ions. A comparison of the sums of mobile fractions extracted using the Pavlotskaya and Tessier methods gives somewhat consistent results: 12.7 and 14.9%, respectively. The share of the fraction bonded with organic matter extracted using the Tessier method is somewhat higher than the share of the acid-soluble fraction extracted according to the Pavlotskaya method. This is because, in the scheme proposed by Pavlotskaya, a portion of 232Th bonded with organic matter was displaced in the preceding fractions. In addition, 232Th is weakly bonded with iron and aluminum compounds moderately deoxidizable and resistant to deoxidization [28].
Unlike 226Ra, the share of 232Th bonded with amorphous iron compounds extracted from a separate soil quantity using the Pavlotskaya method is slightly higher than the sum of mobile fractions extracted using the Tessier method: 16.0 and 14.9%, respectively.
Uranium-238
The sum of the exchangeable and specifically sorbed fractions extracted using the Tessier method is significantly greater than the sum of the water-soluble and exchangeable fractions extracted according to the Pavlotskaya method; this may be caused by the same reasons as in the case of 232Th.
Similarly to 226Ra, the sum of 238U mobile fractions extracted using the Pavlotskaya method (7.3%) is significantly less than the sum of mobile fractions extracted according to the Tessier method (20.2%). Concurrently, the share of 238U bonded with amorphous iron compounds extracted using the Pavlotskaya method (23.4%) is close to the sum of mobile fractions extracted according to the Tessier method (20.2%).
By contrast, the share of the 238U acid-soluble fraction extracted using the Pavlotskaya method is significantly higher than the share of the fraction bonded with organic matter extracted according to the Tessier method. Apparently, this is because the acid-soluble fraction extracted using the Pavlotskaya method contains 238U bonded with iron and aluminum compounds moderately deoxidizable and resistant to deoxidization.
A comparison of the Pavlotskaya and Tessier sequential extraction methods in relation to the fractionation of natural radionuclides (226Ra, 232Th, and 238U) gives poorly consistent results. The Tessier method indicates higher contents of compounds available to plants (the exchangeable and carbonate fraction) in comparison with the Pavlotskaya method (the water-soluble and exchangeable fractions): by 1.8, 3.4, and 3.7 times for 232Th, 238U, and 226Ra, respectively. In a similar way, the Tessier method indicates higher contents of mobile compounds in comparison with the Pavlotskaya method: by 1.2, 2.8, and 3.4 times for 232Th, 238U, and 226Ra, respectively. The biggest differences in the mobility and availability to plants shown by the two methods were noted for 226Ra.
A possible reason behind this is the higher displacing power of divalent Mg2+ ions (MgCl2 in the Tessier method) in relation to multivalent ions in comparison with monovalent \({\text{NH}}_{4}^{ + }\) ions (CH3COONH4 in the Pavlotskaya method). In addition, soluble and poorly soluble 238U and 232Th forms may be represented by complex compounds [17, 28] the stability of which is higher in the neutral magnesium chloride solution in comparison with the dilute acidic ammonium acetate solution. Furthermore, the Tessier method uses hydrochloric hydroxylamine under acidic conditions for extraction of the fraction bonded with Fe and Mn oxides; this results in the fuller extraction of radionuclides bonded with Mn oxides in comparison with the hydrochloric acid treatment used to extract the mobile fraction in the Pavlotskaya method. The higher share of Mn in the fraction bonded with Fe and Mn oxides extracted using the Tessier method (Table 5) in comparison with the mobile fraction extracted according to the Pavlotskaya method (Table 4) confirms this: 40.6 versus 30.0% of the sum, respectively.
CONCLUSIONS
A comparison of the Pavlotskaya and Tessier sequential extraction methods in relation to the fractionation of various forms of occurrence of technogenic radionuclides (137Cs and 90Sr) gives substantially consistent results. The sum of the water-soluble and exchangeable (highly soluble) fractions extracted using the Pavlotskaya method and the sum of the exchangeable and carbonate fractions extracted according to the Tessier method should be used for the assessment of the availability of radionuclides to plants. The sums of the mobile fractions extracted using the Pavlotskaya method (the water-soluble, exchangeable (highly soluble), and mobile proper fractions) and the sum of the exchangeable fraction, carbonate fraction, and the fraction bonded with Fe and Mn oxides extracted according to the Tessier method should be used for the assessment of the geochemical mobility of radionuclides.
The application of the above methods for the speciation assessment of natural radionuclides (226Ra, 232Th, and 238U) gives poorly consistent results. The Tessier method indicates higher contents of compounds available to plants and mobile compounds in comparison with the Pavlotskaya method. The main reason behind this may be the complexity of the soil chemistry of radionuclides such as 232Th and 238U that feature polyvalence and a strong tendency for hydrolysis and complex formation; in addition, their behavior may be affected by various carriers. Therefore, these elements form a broad range of compounds that change one into another with changes in the chemical conditions; this complicates accurate comparison of the composition of their forms extracted by the reagents used in the above methods.
An advantage of the Tessier method is the selective extraction of radionuclides bonded with organic matter, while in the Pavlotskaya method, radionuclides bonded with organic matter constitute parts of the mobile and acid-soluble fractions. On the other hand, the Tessier method does not include the extraction of water-soluble compounds constituting the most available and mobile portion of the soil radionuclide pool. In addition, the Tessier method does not include the extraction of compounds bonded with crystalline iron and aluminum oxides, which makes comprehensive assessment of the behavior of natural radionuclides (226Ra, 232Th, and 238U) impossible.
REFERENCES
Pavlotskaya, F.I., Forms of occurrence and migration of radioactive products of global fallouts in soils, Extended Abstract of Doctoral (Chem.) Dissertation, Moscow: Inst. Geokhim. Anal. Khim. im. V.I. Vernadskogo Akad. Nauk SSSR, 1981.
Goryachenkova, T.A., Kazinskaya, I.E., Novikov, A.P., et al., Comparison of methods for assessing plutonium speciation in environmental objects, Radiochemistry, 2005, vol. 47, no. 6, pp. 599–604.
Tessier, A., Campbell, P.G.C., and Bisson, M., Sequential extraction procedure for the speciation of particulate trace metals, Anal. Chem., 1979, vol. 51, no. 7, pp. 844–851.
Filgueiras, A.V., Lavilla, I., and Bendicho, C., Chemical sequential extraction for metal partitioning in environmental solid samples, J. Environ. Monit., 2002, vol. 4, no. 6, pp. 823–857.
Ladonin, D.V., Heavy metal compounds in soils: problems and methods of study, Eurasian Soil Sci., 2002, vol. 35, no. 6, pp. 605–614.
Clark, S.B., Johnson, W.H., Malek, M.A., et al., A comparison of sequential extraction techniques to estimate geochemical controls on the mobility of fission product, actinide, and heavy metal contaminants in soils, Radiochim. Acta, 1996, vol. 74, no. s1.
Gworek, B. and Mocek, A., Comparison of sequential extraction methods with reference to zinc fractions in contaminated soils, Pol. J. Environ. Stud., 2003, vol. 12, no. 1, pp. 41–48.
Oyeyiola, A.O., Olayinka, K.O., and Alo, B.I., Comparison of three sequential extraction protocols for the fractionation of potentially toxic metals in coastal sediments, Environ. Monit. Assess., 2011, vol. 172, nos. 1–4, pp. 319–327.
Plekhanova, I.O. and Bambusheva, V.A., Extraction methods for studying the fractional composition of heavy metals in soils and their comparative assessment, Eurasian Soil Sci., 2010, vol. 43, no. 9, pp. 1004–1010.
Tasic, A., Sredovic-Ignjatovic, I., Ignjatovic, L., et al., Comparison of sequential and single extraction in order to estimate environmental impact of metals from fly ash, J. Serbian Chem. Soc, 2016, vol. 81, no. 9, pp. 1081–1096.
Tlustoš, P., Száková, J., Stárková, A., and Pavlíková, D., A comparison of sequential extraction procedures for fractionation of arsenic, cadmium, lead, and zinc in soil, Cent. Eur. J. Chem., 2005, vol. 3, no. 4, pp. 830–851.
Blanco, P., Tomé, F.V., and Lozano, J.C., Sequential extraction for radionuclide fractionation in soil samples: a comparative study, Appl. Radiat. Isot., 2004, vol. 61, nos. 2–3, pp. 345–350.
Schultz, M.K., Burnett, W., Inn, K.G.W., and Smith, G., Geochemical partitioning of actinides using sequential chemical extractions: comparison to stable elements, J. Radioanal. Nucl. Chem., 1998, vol. 234, nos. 1–2, pp. 251–256.
Miller, W.P., Martens, D.C., and Zelazny, L.W., Effect of sequence in extraction of trace metals from soils, Soil Sci. Soc. Am. J., 1986, vol. 50, no. 3, p. 598.
Smith, G.E., Fractionation of Actinide Elements in Sediments via an Optimized Protocol for Sequential Extractions, Florida State University, 1998.
Shcheglov, A.I., Biogeokhimiya tekhnogennykh radionuklidov v lesnykh ekosistemakh: po materialam 10-letnikh issledovanii v zone vliyaniya avarii na ChAES (Biogeochemistry of Artificial Radionuclides in Forest Ecosystems Based on the Results of Ten Years of Research in the Impact Area of the Chernobyl Accident), Moscow: Nauka, 2000.
Tyazhelye estestvennye radionuklidy v biosfere: migratsiya i biologicheskoe deistvie na populyatsii i biogeotsenozy (Heavy Natural Radionuclides in the Biosphere: Migration and the Biological Effect on Populations and Biogeocoenoses), Moscow: Nauka, 1990.
Kruglov, S.V., Kurinov, A.D., and Arkhipov, N.P., Forms of occurrence of radionuclides in the soils of the 30-km zone of the Chernobyl Nuclear Power Plant and their change over time, in IV Mezhdunar. nauch.-tekhn. konf. “Itogi 8 let raboty po likvidatsii posledstvii avarii na ChAES”, Sbornik dokladov (Proc. IV Int. Sci.-Techn. Conf. “The Results of Eight Years of Work in the Aftermath of the Chernobyl Accident”), Chernobyl, 1994, vol. 1, pp. 243–250.
Arkhipov, N.P., Fedorova, T.A., and Fevraleva, L.T., Relative amounts of compounds of heavy natural radionuclides, Sov. Soil Sci., 1986, vol. 18, no. 3, pp. 66–70.
Kruglov, S.V., Aleksakhin, R.M., Vasil’yeva, N.A., Kurinov, A.D., and Ratnikov, A.N., Evolution of the radionuclide composition of soils near the Chernobyl Nuclear Power Station, Sov. Soil Sci., 1991, vol. 23, no. 5, pp. 58–66.
Metodika prigotovleniya schetnykh obraztsov prob pochvy dlya izmereniya aktivnosti strontsiya-90 na beta-spektrometricheskikh kompleksakh s programmnym obespecheniem “Progress” (A Method of Preparation of Count Samples of Soil Specimens for Measuring the Activity of Strontium-90 Using Beta-Spectrometer Complexes with the Progress Software), OOO NTTs Amplituda, 1997.
Metodika prigotovleniya schetnykh obraztsov iz prob pit’evoi vody dlya izmereniya aktivnosti ERN s ispol’zovaniem radiologicheskogo kompleksa s programmnym obespecheniem “Progress” (A Method of Preparation of Count Samples of Drinking Water Specimens for Measuring the Activity of Natural Radionuclides Using a Radiological Complex with the Progress Software), OOO NTTs Amplituda, 2006.
Vdovenko, V.M. and Dubasov, Yu.V., Analiticheskaya khimiya radiya (Analytical Chemistry of Radium), Leningrad: Nauka, 1973.
Sanzharova, N.I., Sysoeva, A.A., Isamov, N.N., Aleksakhin, R.M., Kuznetsov, V.K., and Zhigareva, T.L., The role of chemistry in the rehabilitation of agricultural lands affected by radioactive contamination, Ross. Khim. Zh., 2005, vol. 49, no. 3, pp. 26–34.
Konopleva, I.V., Selective sorption of radiocesium by sorbents based on natural clay, Sorbts. Khromatogr. Prots., 2016, vol. 16, no. 4, pp. 446–456.
Bolt, G.H., Sumner, M.E., and Kamphorst, A., A study of the equilibria between three categories of potassium in an illitic soil, Soil Sci. Soc. Am. J., 1963, vol. 27, no. 3, p. 294.
Cremers, A., Elsen, A., De Preter, P., and Maes, A., Quantitative analysis of radiocaesium retention in soils, Nature, 1988, vol. 335, no. 6187, pp. 247–249.
Rachkova, N.G., Shuktomova, I.I., and Taskaev, A.I., The state of natural radionuclides of uranium, radium, and thorium in soils, Eurasian Soil Sci., 2010, vol. 43, no. 6, pp. 651–658.
Funding
This study was supported by the Russian Foundation for Basic Research, project no. 18-04-00584 A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by L. Emeliyanov
Rights and permissions
About this article
Cite this article
Manakhov, D.V., Emelyanov, A.M., Karpukhin, M.M. et al. Comparison of Methods for Assessment of Radionuclide Speciation in Soils. Biol Bull Russ Acad Sci 46, 1671–1678 (2019). https://doi.org/10.1134/S1062359019120057
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359019120057