Abstract
Our previous findings have indicated that Bacillus mucilaginosus might be a promising biosorbent. However, up to now, few studies have been performed to examine the use of B. mucilaginosus as a sorbent, especially as a sorbent for Hg(II). The aim of the current study was to investigate the adsorption of Hg(II) by B. mucilaginosus and the underlying mechanism involved. The results showed that B. mucilaginosus exhibited effective adsorption of Hg(II), and the experimental data were well fitted by the Langmuir model with equilibrium constant of 3.32 × 104 M−1 and maximum adsorption capacity of 393 mg(Hg)/l(bacterial culture). The average saturated adsorption amount of Hg(II) by each cell was 9.83 × 109 atoms, with time to reach adsorption equilibrium less than 10 min. The adsorption efficiency was mainly dependent on pH. Surface adsorption of capsules was identified to be the major mechanism for the biosorption of Hg(II) by B. mucilaginosus, which might be associated with the cell products on the surface of capsules of B. mucilaginosus. Differences observed in adsorption behaviors at different concentrations of Hg(II) were well explained using the Visual minTEQ software. Our findings might shed some lights on the application of B. mucilaginosus as an adsorbent for Hg(II) and other heavy metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The application of biosorption to treat polluted water containing heavy metals has become a hot research topic in recent years. Most biosorption studies have been focused on the adsorption of metals and related substances using microbial systems (Gadd 2009; Ledin 2000). These studies have shown that using of biosorption to remove toxic heavy metals from wastewater has unique advantages (Demirbas 2008; Gadd 2009). Microorganisms have complex structures and diverse cell products, this result the complexity of adsorption processes and diversity of mechanisms, and make the biosorption of heavy metals quite different from conventional physical–chemical adsorption. The studies regarding adsorption of heavy metals by microorganisms have been conducted in the areas of strain screening (Pal et al. 2006; Pumpel et al. 1995; Tsuruta 2006), adsorption efficiency (Gabr et al. 2009; Lopez et al. 2002; Mamba et al. 2009), adsorption mechanisms and models (Gabr et al. 2009; Kularatne et al. 2009), effect factors (Guine et al. 2007; Takenaka et al. 2007; Willow and Cohen 2003), adsorbent immobilization (Gabr et al. 2009; Lopez et al. 2002; Zhou et al. 2009), etc. However, detailed mechanisms for heavy metals adsorption by microorganisms have not been fully studied (Gadd 2009). A variety of models have been used to characterize biosorption of heavy metals, among which the Langmuir and Freundlich models have been widely accepted (Ozdemir et al. 2004; Zhou et al. 2009). The complicated structures and various cell products of microorganisms make their mechanisms and behaviors for adsorption of heavy metals more complex. Moreover, there were marked differences in the biosorption behaviors among different species of microorganisms (Al-Garni 2005; Ginn and Fein 2009), and the functional groups on cell surface involved in biosorption were also diverse (Fowle et al. 2000; Mishra et al. 2009). Many studies have been performed to explore the quantitative and/or qualitative relationships between adsorption efficiency and various factors (Dias et al. 2000; Gorman-Lewis et al. 2005; Kazy et al. 2006; Naeem et al. 2006). However, the quantitative description and interpretation for adsorption parameters are far from perfect.
The elevated Hg(II) level in environment has raised more and more concerns (Johnels and Westermark 1969; Feng and Qiu 2008). However, compared with other toxic heavy metals, not many studies have been carried out regarding Hg removal by microorganisms. Daughney et al. (2002) studied Hg(II) adsorption by Bacillus subtilis and found that the bacterium showed good adsorption of Hg(II) at 20 ppb in solution, which could be described using surface complexation model (SCM). In addition, effects of various factors including bacteria-to-metal ratio, pH, chloride concentration, bacteria growth phase, reaction time, etc., on the adsorption of Hg(II) by B. subtilis were explored.
Bacillus mucilaginosus, namely silicate bacteria, is one of the common soil bacteria, which has been used as a model strain in the research of silicate mineral weathering (Basak and Biswas 2009; Hu et al. 2006; Kupriyanova-Ashina et al. 1998; Malinovskaya et al. 1990). Extensive studies regarding this bacterium have been mainly focused on potassium releasing from soil minerals and its application as a bacterial bio-fertilizer like PGPR (plant growth promoting rhizobacteria) (Basak and Biswas 2009; Lian et al. 2002). In recent years, it has been found that B. mucilaginosus can be used in wastewater treatment to remove various contaminants (Lian et al. 2008; Ye and Bin 2005; Zhao et al. 2008). Existing studies have demonstrated that, as a biosorbent, each species of microorganisms has its own specific adsorption characteristics and mechanism, and the impact of environmental factors, e.g., temperature and pH, on adsorption efficiency, might be quite different. Existing studies showed that B. mucilaginosus was an excellent bacterium strain with good adsorption effect and application prospect in wastewater treatment (Lian et al. 2008; Ye and Bin 2005; Zhao et al. 2008). However, the adsorption mechanism and effects of various environmental factors regarding the adsorption of heavy metals, especially Hg(II), by B. mucilaginosus still need further investigation. In this study, biomass of B. mucilaginosus was used as the biosorbent and HgCl2 was used as the adsorbate to simulate Hg wastewater. The adsorption of Hg(II) by this bacterium was examined, and the adsorption equilibrium constant as well as the adsorption capacity of Langmuir equation under the current experimental conditions were calculated. Further the adsorption mechanism and relevant impact factors on the adsorption were determined.
Materials and methods
Bacterial culture
Bacteria strain (B. mucilaginosus K02, GenBank database accession number: HM579819, stored at Environmental Biological Science and Technology Research Center, Institute of Geochemistry, Chinese Academy of Sciences) was inoculated into 500 ml sterile medium (per 1,000 ml medium containing 5 g sucrose, 5 g Na2HPO4·12H2O, 0.5 g MgSO4·7H2O, 0.1 g calcium carbonate, 1.0 mg ferric chloride, and 0.2 g yeast extract in secondary deionized water) and incubated on a rotary shaker at 150 rpm and 28 ºC for 5 days before use.
Adsorption of Hg(II) by B. mucilaginosus
Adsorption of different concentrations of Hg(II) by given volume of bacterial culture
5 ml of bacterial culture and certain amounts HgCl2 (AR) were added to 250 ml-flasks, followed by adding secondary deionized water to make up the final volume to 100 ml. The initial concentrations of Hg(II) were 0.5, 1, 2, 5, 10, 20, 50, 100, 200, 500 and 1,000 ppm. After 10 min of shaking, the pH values of the solutions were measured (each concentration was studied at its own pH, around 6) using a pH meter (PHS-3C). After standing at 10°C for 24 h (24 h after, pH values were measured again, and the differences were not more than 0.02), the reaction mixtures were centrifuged at 5,000 rpm for 20 min (TDL-5-A centrifuge), and the concentrations of Hg in the supernatants were measured. The experiments of 5 ml sterile medium (free of bacteria) adsorbing Hg (1, 2, 5, 10, 20, 50, 100, ppm, respectively) in 100 ml reaction system were performed as control experiments.
Adsorption of Hg(II) at given concentration by different volumes of bacterial culture
Different volumes of the culture (0.5, 1, 2, 5, 10, 20, 50 and 99.5 ml, respectively) and certain amounts of HgCl2(AR) were added to 250 ml-flasks. The total volume was made up to 100 ml using water with initial Hg(II) concentration of 10 ppm. After 10 min of shaking, the pH values of the solutions were measured (each was studied at its own pH, around 6). Then the solutions were centrifuged at 5,000 rpm for 20 min after standing at 10°C for 24 h (24 h after, pH values were measured again, and the differences were not more than 0.02), and the concentrations of Hg in the supernatants were measured. The experiments of sterile medium (free of bacteria) (1, 2, 5, 10, 20 ml, respectively) adsorbing Hg (10 ppm) in 100 ml reaction system were performed as control experiments.
Effect of adsorptive time on Hg(II) adsorption
Certain amounts of the culture and HgCl2 were added to 250 ml-flasks, resulting in initial Hg(II) concentration of 10 ppm and the culture content of 5% (v/v) in a 100 ml reaction system. After 10 min of shaking, and standing at 10°C for certain time (0, 50 min, 4 h 50 min, and 23 h 50 min, total adsorptive time 10 min, 1, 5, and 24 h, respectively), the solutions were centrifuged at 5,000 rpm for 20 min and the concentrations of Hg in the supernatants were measured.
Measurement of Hg concentration
Hg concentration-absorbance relationship equation
Standard Hg solution was diluted to 0, 2, 4, 6, 8, and 10 ppb, respectively, using potassium dichromate(AR)-nitric acid(GR) solution. The absorbance of each standard solution was measured according to Luo et al.’s method (2005) using F732-S mercury analyzer with SnCl2 (10%) solution as reducing agent, with repeated measurements for samples of 0, 2, 4, 8 and 10 ppb and seven times of measurements for sample of 6 ppb according to the instrument manual. The Hg concentration-absorbance relationship equation was obtained.
Measurement of Hg concentration in the supernatants
The samples were diluted with potassium dichromate(AR)-nitric acid(GR) solution to result in Hg concentrations in the range of 2–10 ppb, and the absorbances were measured twice for each sample. Hg concentrations (C, equilibrium concentrations in the supernatants) were calculated according to the Hg concentration-absorbance relationship equation, and then the adsorbed Hg concentrations were calculated as C0-C, here C0 were the initial Hg concentrations.
Effect of pH on adsorption
Hg(II) Adsorption ratios at different pH values
Certain amounts of the culture and HgCl2 were added to 250 ml-flasks, resulting in initial Hg(II) concentration of 10 ppm and the culture content of 5% (v/v) in a 100 ml reaction system. The pH was adjusted to 3, 5, 7, 9, and 11, respectively using HCl(AR)(0.185 M) or KOH(AR)(0.20 M) solution, and was monitored till pH value remained constant at given value. After 10 min of shaking, and standing at 10°C for 24 h (24 h after, pH values were measured again, and the differences were not more than 0.05), the solutions were centrifuged at 5,000 rpm for 20 min and the concentrations of Hg in the supernatants were measured.
pH of HgCl2 solutions at different concentrations
Two groups of HgCl2 solutions with different Hg concentrations (0.5, 1, 2, 5, 10, 20, 50, 100, 200, 500, and 1,000 ppm) were prepared. One group contained 5% (v/v) the culture while the other did not. The pH values of the solutions were measured.
Acid-base titration curve of the culture
5 ml culture was mixed with 95 ml secondary deionized water, followed by the addition of HCl(AR) solution (0.185 M) or KOH(AR) solution (0.2 M) solution drop by drop. The resulting pH values were monitored.
GC–MS analysis of volatile components in the culture
Volatile components in the culture were extracted with ether and analyzed using HP6890/HP5973 GC/MS (Hewlett Packard, USA) (Zhou et al. 2004). The contents of different volatile compounds were calculated by the method of peak area normalization.
Bacterial concentration in the culture
Direct counting method under a microscope (PH2000-type, Fenghuang, China) was used to determine bacterial concentration in the culture.
Proportion of complexes distribution of Hg(II) at different concentrations
Visual minTEQ water chemistry calculation software was used to calculate the proportion of various forms of Hg(II) complexes at different concentrations.
Results and discussion
Hg concentrations-absorbances relationship equation
The experimental results showed a good linear relationship between Hg concentrations (0–10 ppb) and the absorbances as follow
Here, Y was measured Hg concentration, and X was absorbance.
Adsorption of Hg at different concentrations by the culture of B. mucilaginosus
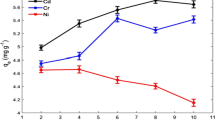
Adsorption ratio A, the removal efficiency of Hg from the solutions, was calculated as A = (C0–C)/C0 × 100% and adsorption capacities q, the amount of adsorbed Hg(mg) by 1 l bacterial culture, was determined as q = V0 × (C0–C)/V mg/l, here V0 was the total volume of the reaction solution (100 ml), V was the volume of the culture (5 ml), C was the equilibrium Hg concentration, and (C0-C) was adsorbed Hg concentration. Figure 1 shows the changed curves of A and q versus initial Hg concentration (C0).
The curves in Fig. 1 indicated that when the culture volume (V) was fixed at 5 ml, A was higher (>98%) and q was lower at lower concentrations of Hg. In this case, the bacterial adsorption sites were far more than the amount of Hg complexes. Thus the majority of Hg complexes were adsorbed, while the bacterial adsorption sites were far from being saturated. In contrast, at higher concentrations of Hg, A was lower and q was higher (374 mg/l, maximum). In this case, bacterial adsorption sites were far less than the amount of Hg. Thus the majority of adsorption sites were occupied by Hg complexes, while a large amount of Hg has not been adsorbed. The results showed that as Hg(II) concentration increased q increased; rapidly at lower levels of Hg(II), and it tended to be saturated at higher levels of Hg, which suggested that the adsorption curve was in accordance with the Langmuir model. Control experiment showed that sterile medium (free of bacteria) could not adsorb mercury at various concentrations. pH values of experimental solutions were at around 6, and decreased gradually with the increasing concentrations of mercury.
Adsorption of Hg(II) by different volumes of bacterial culture
The change trends of A and q with the cultural volume as showed in Fig. 2 were opposite to the trends observed in Fig. 1. Control experiment showed that sterile medium of various concentrations (free of bacteria) could not adsorb mercury. pH values of experimental solutions were at around 6, and increased gradually with the increasing volumes of bacterial culture.
Effect of time on adsorption
The adsorption ratios were close among different adsorption times (43.9, 44.6, 43.1, and 45.3%, for 10 min, 1, 5, and 24 h, respectively), which indicated that the bacterium had a comparatively rapid adsorption of Hg and the adsorption equilibrium could be reached within 10 min. It has been reported that adsorption of heavy metals by microorganisms usually reach equilibrium within 5 min–4 h (Fan et al. 2007; Green-Ruiz 2006; Lopez et al. 2002; Zhou et al. 2009). Considering the bacterium had thick capsules on the cell surfaces, the adsorption rate was very high, and the basic assumption of Langmuir model is monolayer adsorption, we propose that the adsorption might be largely surface adsorption by those capsules.
Compared with other microorganisms, e.g., B. subtilis, which adsorbs metals by cell walls, adsorption of Hg by capsules might be a prominent characteristic of B. mucilaginosus as a biosorbent. Capsules could improve bacterial adsorption capacity (Al-Garni 2005) and might have higher tolerance to toxic heavy metals (Malik 2004; Yilmaz 2003).
Effect of pH on adsorption
pH of HgCl2 solution at different concentrations
To better understand the mechanism for the adsorption, the pH of HgCl2 solutions at different concentrations were measured. The results showed that the pH of HgCl2 solutions decreased with increasing HgCl2 concentrations. The pH values of the HgCl2 solutions containing the culture remained stable when the concentration of Hg was less than 5 ppm, and then decreased with increasing HgCl2 concentrations. This might be because that HgCl2 is a strong acid-weak base salt, and the bacterial culture possessed some buffer capacity.
Effect of pH on the adsorption
The effect of pH on the adsorption of Hg(II) by the bacterium is shown in Fig. 3, the data indicated that pH had a great impact on the adsorption ratio A, which increased with increasing pH. This might be due to the occupation of adsorption sites by protons when pH decreased. No dramatic changes were observed for the adsorption ratio when pH was altered, except that changes were rapid in some regions while slow in others. This might be related to the diversity of cell products on the surface of capsules, and the variety of cell products in properties and contents.
Acid-base titration curve of the culture
Figure 4 indicated that the curve had a rapid change region when the pH was within 4–6, which might be related to the effect of acetic acid, which has a pKa of 4.75. The other region with rapid change was within 8–9, which might be associated with 1, 3-dihydroxy-2-propanone and acetol. The contents of the above three components were obviously higher than other metabolites.
GC–MS analysis of volatile components in the culture
The results demonstrated that there were numerous organic acids, alcohols, and ketones in the culture. The volatile components with relative contents more than 10% included acetic acid (17.30%), 1, 3-dihydroxy-2-propanone (22.11%) and acetol (11.67%). In addition, the bacterial culture also contained large amounts of polysaccharides (Lian et al. 2008), which might play a great role in the adsorption of Hg by the bacterial biomass. The culture contained large amount of acetate anion under the current experimental pH conditions, and acid-base titration curve of the culture had obvious changes around the pKa (Fig. 4) of acetic acid, which suggested that the changes of the pH and adsorption ratios of Hg might be related to the transformation of acetic acid existence form and the formation of Hg complexes with acetate. At higher pH, hydrolysis might occur for 1, 3-dihydroxy-2-propanone and acetol, which resulted the formation of complexes with Hg(II) and further increase of A. The high content of cell products, e.g., acetic acid, 1, 3-dihydroxy-2-propanone, and acetol, etc., in the culture, might play a great role in metal adsorption.
Calculation of Langmuir model parameters and the adsorption capacity by a single cell
Based on the experimental data, Langmuir adsorption constants were calculated according to the following formula
In formula (1), q was adsorption capacity (adsorbed Hg (mg) by 1 l the bacterial culture, because of the high viscosity of the culture, it was difficult to determine dry weight of the biomass accurately, so we reported the maximum adsorption capacity in mg(Hg)/l), q 0 was maximum adsorption capacity, k was the adsorption equilibrium constant, and C was the adsorption equilibrium concentration. The formula (1) was transformed into:
There was a linear relationship between C/q and C, with the slope of 1/q 0 and the intercept of 1/q 0 k.
By comparing experiment data with calculated values repeatedly, basing on six sets of experimental data at high concentrations, the calculated results were consistent with the experimental data as follows (Fig. 5):
The bacterial concentration in the culture was 1.2 × 1011 cells/l, and the theoretical calculation value q 0 was 393 mg(Hg)/l(the culture). Thus, according to the Avogadro constant and the atomic weight of Hg, each cell could adsorb 9.83 × 109 Hg atoms.
The results indicated that B. mucilaginosus was an effective biosorbent for Hg(II) at comparatively higher Hg levels, and the adsorption behavior was well fitted with the Langmuir model. Equilibrium adsorption constant was 3.32 × 104 M−1, and maximum adsorption capacity could reach 393 mg(Hg)/l (the bacterial culture), i.e., averagely each bacterial cell could maximumly adsorb 9.83 × 109 Hg atoms. The dominated existence forms of Hg(II) in the solution were neutral complexes of Hg(OH)2(aq) and HgCl2(aq), which were likely to affect bacterial adsorption. The bacterium might have even better adsorption efficiency for other heavy metals existing as ionic forms, since there would be stronger electrostatic forces between metal ions and polar functional groups.
Analysis of differences between experimental data and calculated values at lower Hg levels
We found that the calculated values of q were well consistent with the experimental values at higher concentrations, but not at lower concentrations. The reason might be that the concentration of HgCl2 exhibited some effects on the adsorption ratio of Hg such as: (1) the pH of HgCl2 solutions were higher at lower concentrations, (2) there were significant differences in the distribution of Hg(II) complexes (Table 1).
In this study, Langmuir adsorption constant and maximum adsorption capacity were obtained basing on experimental values at higher concentrations, so the calculated values were well consistent with experimental data at higher concentrations. The changes of distribution of Hg(II) complexes and comparatively higher pH at lower concentrations resulted in higher experimental values than the calculated ones. How to modify Langmuir equation obtained at higher concentrations to fit the adsorption at lower concentrations still needs to be further studied.
Conclusions
Our study showed that B. mucilaginosus was an effective biosorbent for Hg(II), and the adsorption behavior was fitted well using the Langmuir model. The adsorption of metals by B. mucilaginosus was mainly attributed to the capsules on cell surface, which could improve bacterial adsorption capacity, and enable the bacteria to have a higher tolerance to toxic heavy metals. The adsorption equilibrium could be reached within 10 min. The pH had a major impact on the adsorption ratio and the concentration of HgCl2 also had some effects on the adsorption due to the differences in the distribution of Hg(II) complexes and the changes of pH values. The adsorption mechanism was largely surface adsorption, and might be closely related to the high content of cell products.
References
Al-Garni SM (2005) Biosorption of lead by gram-ve capsulated and non-capsulated bacteria. Water Sa 31:345–349
Basak BB, Biswas DR (2009) Influence of potassium solubilizing microorganism (Bacillus mucilaginosus) and waste mica on potassium uptake dynamics by sudan grass (Sorghum vulgare Pers.) grown under two alfisols. Plant Soil 317:235–255. doi:10.1007/s11104-008-9805-z
Daughney CJ, Siciliano SD, Rencz AN, Lean D, Fortin D (2002) Hg(II) adsorption by bacteria: a surface complexation model and its application to shallow acidic lakes and wetlands in kejimkujik national park, nova scotia, Canada. Environ Sci Technol 36:1546–1553. doi:10.1021/es010713x
Demirbas A (2008) Heavy metal adsorption onto agro-based waste materials: a review. J Hazard Mater 157:220–229. doi:10.1016/j.jhazmat.2008.01.024
Dias MA, Castro HF, Pimentel PF, Gomes NCM, Rosa CA, Linardi VR (2000) Removal of heavy metals from stainless steel effluents by waste biomass from Brazilian alcoholic beverage production. World J Microbiol Biotechnol 16:107–108
Fan RM, Zhang BG, Zhang HX, Fan JH, Wang Q, Bai ZH (2007) Study on adsorption of Zn2+ by Bacillusclausii S-4. Chin J Environ Eng 1:44–47 (in Chinese, with English abstract)
Feng XB, Qiu GL (2008) Mercury pollution in guizhou, southwestern China—an overview. Sci Total Environ 400:227–237. doi:10.1016/j.scitotenv.2008.05.040
Fowle DA, Fein JB, Martin AM (2000) Experimental study of uranyl adsorption onto Bacillus subtilis. Environ Sci Technol 34:3737–3741. doi:10.1021/es991356h
Gabr RM, Gad-Elrab SMF, Abskharon RNN, Hassan SHA, Shoreit AAM (2009) Biosorption of hexavalent chromium using biofilm of E. coli supported on granulated activated carbon. World J Microbiol Biotechnol 25:1695–1703. doi:10.1007/s11274-009-0063-x
Gadd GM (2009) Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J Chem Technol Biotechnol 84:13–28. doi:10.1002/jctb.1999
Ginn B, Fein JB (2009) Temperature dependence of Cd and Pb binding onto bacterial cells. Chem Geol 259:99–106. doi:10.1016/j.chemgeo.2008.09.021
Gorman-Lewis D, Elias PE, Fein JB (2005) Adsorption of aqueous uranyl complexes onto Bacillus subtilis cells. Environ Sci Technol 39:4906–4912. doi:10.1021/es047957c
Green-Ruiz C (2006) Mercury(II) removal from aqueous solutions by nonviable Bacillus sp. from a tropical estuary. Bioresour Technol 97:1907–1911. doi:10.1016/j.biortech.2005.08.014
Guine V, Martins JMF, Causse B, Durand A, Gaudet JP, Spadini L (2007) Effect of cultivation and experimental conditions on the surface reactivity of the metal-resistant bacteria Cupriavidus metallidurans CH34 to protons, cadmium and zinc. Chem Geol 236:266–280. doi:10.1016/j.chemgeo.2006.10.001
Hu XF, Chen JS, Guo JF (2006) Two phosphate- and potassium-solubilizing bacteria isolated from Tianmu mountain, Zhejiang, China. World J Microbiol Biotechnol 22:983–990. doi:10.1007/s11274-006-9144-2
Johnels AG, Westermark T (1969) Mercury contamination of the environment in Sweden. Chem Fall Curr Res Pers Pestic 221–241
Kazy SK, Das SK, Sar P (2006) Lanthanum biosorption by a Pseudomonas sp.: equilibrium studies and chemical characterization. J Ind Microbiol Biotechnol 33:773–783. doi:10.1007/s10295-006-0108-1
Kularatne RKA, Kasturiarachchi JC, Manatunge JMA, Wijeyekoon SLJ (2009) Mechanisms of manganese removal from wastewaters in constructed wetlands comprising water hyacinth (Eichhornia crassipes (Mart.) Solms) grown under different nutrient conditions. Water Environ Res 81:165–172. doi:10.2175/106143008X370403
Kupriyanova-Ashina FG, Krinari GA, Kolpakov AL, Leschinskaya IB (1998) Degradation of silicate minerals by Bacillus mucilaginosus using bacillus intermedius RNase. Towards Sustain Land Use 31:813–818 Vols I & Ii
Ledin M (2000) Accumulation of metals by microorganisms-processes and importance for soil systems. Earth-Sci Rev 51:1–31. doi:10.1016/S0012-8252(00)00008-8
Lian B, Souleimanov A, Zhou X, Smith DL (2002) In vitro induction of lipo-chitooligosaccharide production in bradyrhizobium japonicum cultures by root extracts from non-leguminous plants. Microbiol Res 157:157–160. doi:10.1078/0944-5013-00145
Lian B, Chen Y, Zhao J, Teng HH, Zhu L, Yuan S (2008) Microbial flocculation by Bacillus mucilaginosus: applications and mechanisms. Bioresour Technol 99:4825–4831. doi:10.1016/j.biortech.2007.09.045
Lopez A, Lazaro N, Morales S, Marques AM (2002) Nickel biosorption by free and immobilized cells of Pseudomonas fluorescens 4F39: a comparative study. Water Air Soil Pollut 135:157–172. doi:10.1023/A:1014706827124
Luo GB, Chen CJ, Gao JF, Wang M, Xie J (2005) Microwave digestion of sewage sample for determination of mercury. J Environ Health 22:477–478 (in Chinese, with English abstract)
Malik A (2004) Metal bioremediation through growing cells. Environ Int 30:261–278. doi:10.1016/j.envint.2003.08.001
Malinovskaya IM, Kosenko LV, Votselko SK, Podgorskii VS (1990) Role of Bacillus-Mucilaginosus polysaccharide in degradation of silicate minerals. Microbiology 59:49–55
Mamba BB, Dlamini NP, Nyembe DW, Mulaba-Bafubiandi AF (2009) Metal adsorption capabilities of clinoptilolite and selected strains of bacteria from mine water. Phys Chem Earth 34:830–840. doi:10.1016/j.pce.2009.07.010
Mishra B, Boyanov MI, Bunker BA, Kelly SD, Kemner KM, Nerenberg R, Read-Daily BL, Fein JB (2009) An X-ray absorption spectroscopy study of Cd binding onto bacterial consortia. Geochim Cosmochim Acta 73:4311–4325. doi:10.1016/j.gca.2008.11.032
Naeem A, Woertz JR, Fein JB (2006) Experimental measurement of proton, Cd, Pb, Sr, and Zn adsorption onto the fungal species Saccharomyces cerevisiae. Environ Sci Technol 40:5724–5729. doi:10.1021/es0606935
Ozdemir G, Ceyhan N, Ozturk T, Akirmak F, Cosar T (2004) Biosorption of chromium(VI), cadmium(II) and copper(II) by Pantoea sp. TEM. Chem Eng J 102:249–253. doi:10.1016/j.cej.2004.01.032
Pal A, Ghosh S, Paul AK (2006) Biosorption of cobalt by fungi from serpentine soil of Andaman. Bioresour Technol 97:1253–1258. doi:10.1016/j.biortech.2005.01.043
Pumpel T, Pernfuss B, Pigher B, Diels L, Schinner F (1995) A rapid screening method for the isolation of metal-accumulating microorganisms. J Ind Microbiol 14:213–217. doi:10.1007/BF01569930
Takenaka Y, Saito T, Nagasaki S, Tanaka S, Kozai N, Ohnuki T (2007) Metal sorption to Pseudomonas fluorescens: influence of pH, ionic strength and metal concentrations. Geomicrobiol J 24:205–210. doi:10.1080/01490450701457337
Tsuruta T (2006) Removal and recovery of uranium using microorganisms isolated from Japanese uranium deposits. J Nucl Sci Technol 43:896–902
Willow MA, Cohen RRH (2003) pH, dissolved oxygen, and adsorption effects on metal removal in anaerobic bioreactors. J Environ Qual 32:1212–1221
Ye C, Bin L (2005) Study on the flocculability of chromium ion by bacillus Mucilaginosus GY03 strain. Pedosphere 15:225–231
Yilmaz EI (2003) Metal tolerance and biosorption capacity of Bacillus circulans strain EB1. Res Microbiol 154:409–441. doi:10.1016/S0923-2508(03)00116-5
Zhao HX, Lian B, Xie ZH, Chen Y, Zhu LJ (2008) Water quality analysis and microbial treatment of the colliery area of Kaili in Guizhou province China. Acta Mineral Sin 28:71–76 (in Chinese, with English abstract)
Zhou X, Li ZW, Wang DP, Liang GY, Peng BX (2004) Study on fingerprint of volatile oil of Curcuma wenyujin by GC-MS. China J Chin Mater Med 29:1138–1141 (in Chinese, with English abstract)
Zhou LC, Li YF, Bai X, Zhao GH (2009) Use of microorganisms immobilized on composite polyurethane foam to remove Cu(II) from aqueous solution. J Hazard Mater 167:1106–1113. doi:10.1016/j.jhazmat.2009.01.118
Acknowledgments
This research was financially supported by the Knowledge Innovational Program of Chinese Academy of Sciences (kzcx2-yw-135-2) and the National Science Fund for Creative Research Groups (40721002). The authors would like to extend their gratitudes to Prof. Hailiang Dong of Miami University for his helpful suggestions and Associate Prof. H. Henry Teng of George Washington University for his great comments for this article. We thank two anonymous reviewers for their insightful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mo, BB., Lian, B. Hg(II) adsorption by Bacillus mucilaginosus: mechanism and equilibrium parameters. World J Microbiol Biotechnol 27, 1063–1070 (2011). https://doi.org/10.1007/s11274-010-0551-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0551-z