Abstract
One novel styrylpyridine derivatives(AV-45) coupled with 99mTc complex was synthesized. 99mTc-BAT-AV-45 was prepared by a ligand exchange reaction employing sodium glucoheptonate, and effects of the amount of ligand, stannous chloride, sodium glucoheptonate and pH value of reaction mixture on the radiolabeling yield were studied in details. Quality control was performed by thin layer chromatography and high performance liquid chromatography. Besides the stability, partition coefficient and electrophoresis of 99mTc-BAT-AV-45 were also investigated. The results showed that the average radiolabeling yield was (95 ± 1%) and 99mTc-BAT-AV-45 with suitable lipophilicity was stable and uncharged at physiological pH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a form of dementia with progressive memory loss, irreversible cognitive decline, language impairment, and behavioral changes. The accumulation of β-amyloid (Aβ) aggregates in the brain is a defining pathologic feature for this disease [1, 2]. Nowadays, clinical diagnosis of AD is primarily done by assessment of clinical symptoms, which is often difficult and unreliable, and the only definitive diagnosis is established by pathological examination of the postmortem staining of affected brain tissue. Single photoemission tomography (SPECT) or positron emission tomography (PET) imaging of β-amyloid plaques in the living human brain may serve as a helpful tool for the early diagnosis of AD and monitoring of therapeutic effects.
Technetium-99m (t1/2 = 6 h, 140 keV) is the most commonly used radionuclide in routine clinical nuclear medicine. Because 99mTc can be easily prepared with a 99Mo/99mTc generator, emits the medium γ-ray energy suitable for γ-camera detection and has the ideal physical half-life compatible with biological localization and residence time required for imaging [3]. New 99mTc-labeled Aβ imaging agents will have more widespread clinical applicability for detecting and eventually quantifying β-amyloid plaques in living brain tissue.
The successful development of [Tc-99m]TRODAT-1 already showed the probability of 99mTc imaging agent that can penetrate the blood–brain barrier(BBB) via a simple diffusion mechanism and localize at sites in the central nervous system [4–6]. Based on this success, many efforts have been made so far to create a suitable Aβ plaques ligand based on 99mTc, but no clinical study of them has been reported [7–16].
(E)-2-(2-(2-(2-[18F]Fluoroethoxy)ethoxy)ethoxy)-5-(4-methylaminostyryl)pyridine ([18F]AV-45) with styrylpyridine core, and high affinity for Aβ plaques (Ki = 2.87 nM), which binds specifically to fibrillar Aβ and has favorable pharmacokinetic properties [17]. Preliminary clinical studies in patients with diagnosed mild AD showed notable retention of [18F]AV-45 in the cortex, known to contain large amounts of amyloid deposits in AD [18, 19]. To develop more useful 99mTc-labeled probes for clinical diagnosis, we synthesized AV-45 derivatives with bis-amino-bis-thiol (BAT).We selected BAT as chelation ligands taking into consideration the permeability of BBB with forming an electrically neutral complex with 99mTc [19].
Here, we report the synthesis of the precursor for labeling by coupling styrylpyridine derivatives(AV-45) with N1,N2-[bis(4-methoxybenzyl) thioethyl] ethanediamine (MBz- BAT),the final free thiol ligands (BAT-AV-45) were obtained by deblocking the 4-methooxybenzyl protecting group with Hg(OAc)2 (Scheme 1). Radiolabeling of BAT-AV-45 was carried outby using stannous chloride method. The quality control was determined by using thin layer chromatography and radio high performance liquid chromatography. The stability, partition coefficient and electrophoresis of 99mTc-BAT-AV-45 were investigated.
Experimental
Materials
All reagents were obtained commercially and used without further purification unless otherwise indicated.(E)-2-(2-(2-(5-(4-(tert-butoxycarbonyl(methyl)amino)styryl)pyridine-2-yloxy)-ethoxy)ethoxy)ethyl-4-methylbenzenesulfonate(Boc-AV-45-OTs) [20] and N1,N2-[bis(4-methoxybenzyl) thioethyl]ethanediamine(MBz-BAT) were self-synthesized. Na99mTcO4 was supplied by Jiangsu Institute of Nuclear Medicine. Electron spray ion(ESI) mass spectra was measured using a Waters Platform ZMD4000LC/MS. NMR spectra was obtained on a Bruker DRX-500 spectrometer, and the chemical shift value was given relative to the internaltetramethylsilane (TMS). Wizard 1470 automatic gamma counter equipped with a multi-channel analyzer (U.S. Perkin Elmer Company). RHPLC was performed on a Waters 600-type high-performance liquid chromatography (the United States Waters Corporation) equipped with a dural λ absorbance detector (Waters 2487), binary HPLC pump (Waters 1525) and Cd (Te) detector equipped with a flow scintillation analyzer (Perkin Elmer).
Synthesis of BAT-AV-45 ligand
(E)-2-(2-(2-(2-(N-(4-methoxybenzylthio)-(N-(N-(4-methoxybenzylthioethyl)aminoethyl))aminoe-thoxy)ethoxy)ethoxy)-5-(4-tert-butoxycarbonylmethylaminostyryl)pyridin(MBz-BAT-AV-45, 1).
Boc-AV-45-OTs (0.21 g, 0.4 mmol) and N1,N2-[bis(4-methoxybenzyl) thioethyl] ethanediamine(MBz-BAT) (0.19 g, 0.45 mmol) were dissolved in anhydrous DMF(10 mL), Potassium carbonate(0.12 g, 0.81 mmol) and Potassium iodide(0.07 g, 0.09 mmol) were added. The reaction mixture was stirred at 60 °C for 24 h. After the evaporation of the solvent, water was added. The mixture was extracted with ethyl acetate. The organic layers were combined and dried with Na2SO4. Evaporation of the solvent gave a residue, which was purified by silica gel chromatography(methanol/methylene chloride/ammonia water;1:20:0.05;V/V/V) to give MBz-BAT-AV-45(0.18 g, 62%). 1H-NMR (500 MHz, CDCl3)δ:8.16(1H, s), 7.76(1H, d, d), 7.42(2H, d), 7.22(6H, m), 6.95(2H, s), 6.81(4H, m), 6.79(1H, d), 4.46(2H, m), 3.81(2H, m), 3.77(1H, m), 3.75–3.60(13H, m), 3.49(2H, t), 2.99(3H, s), 2.95(1H, br), 2.79(6H, br), 2.69(2H, t), 2.63(2H, t), 2.57(2H, t), 1.47(9H, s); MS(ESI):positive mode m/z = 862([M + 1]+).
(E)-2-(2-(2-(2-(N-mercaptoethyl)-(N-(N-mercaptoethylaminoethyl))aminoethoxy)ethoxy)ethoxy)-5-(4-methylaminostyryl)pyridin(BAT-AV-45, 2).
MBz-BAT-AV-45(0.18 g, 0.2 mmol) was dissolved in TFA (10 mL) and anisole (0.05 mL, 0.46 mmol) at 0 °C, and Hg(OAc)2 (0.39 g, 1.22 mmol) was added. The resulting mixture was stirred for 30 min and concentrated in vacuo to obtain a viscous oil that was dried in vacuo for 30 min. Dry ether (10 mL) was then added to the above oil, and the resulting suspension was sonicated for 5 min. The yellow solid that formed was collected by suction filtration, dried in vacuo for 20 min, and dissolved in absolute EtOH (10 mL). H2S gas was passed through the solution for 20 min, and the reaction mixture was filtered through a pad of Celite. The filtrate was concentrated in vacuo to obtain the trifluoroacetate salts of BAT-AV-45, which were used for further reactions without additional purification. MS(ESI):positive mode m/z = 521([M + 1]+).
Radiolabeling procedure
In the labeling of 99mTc-BAT-AV-45, BAT-AV-45(50 μg) was dissolved in 100 μL ethanol in an evacuated nitrogen filled vial. To this solution, sodium glucoheptonate (10 mg) dissolved in 0.2 mL water, stannous chloride (50 μg) dissolved in HCl(0.1 N) were added respectively, pH value(5–6) of the solution was adjusted by using HCl or NaOH. After addition of all reagents, 0.1 mL of [Tc-99 m]pertechnetate solution(~0.5 mCi) and additional some water were added into the vial. Reaction mixture volume used was about 0.9 mL. The vial was incubated at 90 °C in a water bath for 30 min. After cooling down to room temperature, 0.1 mL of injected sodium phosphate solution (0.1 M, pH 7.4) was added. A series of studies were performed to optimize labeling efficiency step by step, such as the amount of BAT-AV-45 ligand, reductant SnCl2, sodium glucoheptonate and the pH value of the reaction mixture.
Quality control of 99mTc-BAT-AV-45
The labeling yield and radiochemical purity were determined by thin layer chromatographic method using polyamide strip. The reaction product was spotted on polyamide strips and developed in methanol/ethyl acetate(1:1; V:V) solution. After developing, the strips were dried at room temperature. Then, they were cut into 0.5 cm pieces and counted by using an automatic γ counter. Retention factor (Rf) and labeling yield were determined from TLC chromatogram data. For comparison, samples of Na99mTcO4, 99mTc-GH and reduced/hydrolyzed 99mTc(RH-99mTc) were also run under identical conditions.
99mTc-BAT-AV-45 was further confirmed by radioHPLC system. The flow rate was maintained at 0.8 mL/min after an injection 10 μL from the reaction mixture into analytical reversed-phase column (Amethyst C18, 4.6 × 150 mm 5 μm, Sepax Technologies, Inc.). The eluting solvent consisted of H2O/acetonitrile (1:4;V:V),and the elution was monitored by observing the radioactivity profile. For comparison, samples of Na99mTcO4, 99mTc-GH were also run under identical condition. The chromatograms for Na99mTcO4, 99mTc-GH, 99mTc-BAT-AV-45 are showing in Fig. 2.
Stability of 99mTc-BAT-AV-45
The stability of freshly prepared 99mTc-BAT-AV-45 at physiological pH was measured at different times at room temperature (~25 °C).
Partition coefficient
A 25 μL aliquot of 99mTc-BAT-AV-45 was added to a test tube containing 3 mL of 1-octanol and 3 mL of 0.02 M phosphate buffer pH 7.4. The test tube was vortexed at room temperature for 5 min and then centrifuged at 3,500 rpm for 10 min. A 100 μL aliquot was taken from the 1-octanol phase and a 100 μL aliquot from the aqueous phase, taking care to avoid cross contamination between the phases and weighed. The radioactivity of the aliquots was counted using an automatic γ counter and the partition coefficient P was calculated using the following equation:
with cpm = counts per minute.
Experiments were performed at least in triplicate.
Electrophoresis
A 5 μL was spotted on a paper strip (3.5 × 13 cm, Whatman 1 chromatography paper) wetted with a mixture of 0.02 M phosphate buffer pH 7.4 and methanol (50:50, V/V). Electrophoresis was performed during 60 min using the described mixture as the electrolyte solution and an applied voltage of 300 V. After drying, the paper was cut into 0.5 cm strips and the activity on each strip was counted using an automatic γ counter.
Results and discussion
Synthesis and radiolabeling procedure
As shown in Scheme 1, the labeling precursor was synthesized by coupling styrylpyridine derivatives(AV-45) with N1,N2-[bis(4-methoxybenzyl) thioethyl] ethanediamine (MBz- BAT), and then deblocking the protecting group. The key compound MBz-BAT-AV-45 was identified by 1H NMR and MS. The labeling precursor BAT-AV-45 was not additionally purified because of its easy oxidation and just identified by MS.
99mTc-BAT-AV-45 was prepared by a ligand exchange reaction employing the precursor 99mTc-GH. Several reaction parameters were optimized by varying relative reaction parameter step by step, such as the amount of BAT-AV-45 ligand, reductant SnCl2, sodium glucoheptonate and the pH value of the reaction mixture.
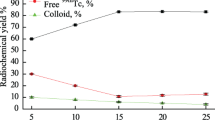
The effects of the amount of BAT-AV-45 ligand were summarized in Fig. 1. The data showed that the radiolabeling yield of 99mTc-BAT-AV-45 reached to 95.7 ± 1% at the optimum amount of ligand (50 μg). Below the amount of 50 μg, the labeling yield was low due to the substrate concentrations being insufficient to form complex with all of the reduced technetium.
The effects of the amount of stannous chloride were shown in Fig. 1. The data showed that the radiolabeling yield was dependent on the amount of stannous chloride, and the highest labeling efficiency was 95.4 ± 1% by using 50 μg of SnCl2. Below the amount of 50 μg, stannous chloride was not sufficient for complete reduction of pertechnetate to form 99mTc-complex because of easy oxidation. Above the amount of 50 μg, the radiochemical yield was decreased. This may be due to the fact that the excess amount of stannous chloride leaded to the formation of stannous hydroxide colloid in basic medium or by oxidation and stannous hydroxide colloid adsorbed the free technetium-99 m or 99mTc-complex.
The effects of the amount of sodium glucoheptonate were summarized in Fig. 1. The data interestingly showed that large amount of sodium glucoheptonate (10 mg) was required for optimal preparation of 99mTc-BAT-AV-45 (RCP .95%), contrast to a minimal amount of ligand. A small amount of sodium glucoheptonate played a transitional ligand role as the form of 99mTc-GH in the radiolabeling procedure. While excess of glucoheptonate with a multi- hydroxyl moiety may facilitate the solubility of BAT-AV-45 ligand by dispersing the compound into reaction solution. Thus 99mTc-GH complex may trans-chelate more effectively with BAT-AV-45 ligand to form 99mTc-BAT-AV-45. Similar results were previously reported in the preparation of [Tc-99m]TRODAT-1 [21].
The effects of pH value were shown in Fig. 1. The data showed that the radiolabeling yield was dependent on the pH value, and the highest labeling efficiency was 95.5 ± 1% with the pH value (5–6). Above the optimum pH value, the radiochemical yield was drastically decreased because of forming RH-99mTc which is the main radiochemical impurities at alkaline medium.
Quality control of 99mTc-BAT-AV-45
In thin layer chromatographic method using methanol/ethyl acetate(1:1;V:V) as solvent, The Rf value of 99mTc-BAT-AV-45 was 0.7–1, and the Rf value of 99mTc-GH was 0–0.2, while RH-99mTc and free Technetium-99m remained at the point of spotting (Rf = 0).The average radiolabeling yield was 95 ± 1%.
HPLC radiochromatograms of Na99mTcO4, 99mTc-GH, 99mTc-BAT-AV-45 are presented in Fig. 2. The radio peak at 2.7 and 4.7 min represent the free Na99mTcO4 and 99mTc-GH respectively. 99mTc-BAT-AV-45 gives one radio peak at 7 min of retention.
Stability of 99mTc-BAT-AV-45
To avoid the radio decomposed side products which may accumulate in non-target organs, the stability of 99mTc-BAT-AV-45 at physiological pH was studied in order to determine the suitable time for injection. The results of the stability of 99mTc-BAT-AV-45 are presented in Fig. 3 . The data clearly shows that 99mTc-BAT-AV-45 is stable (RCP. > 90%) for up to 6 h which is suitable for nuclear medicine applications.
Partition coefficient
Previous studies suggested that the optimal lipophilicity for entry into the brain is obtained with log P values of between 1 and 3 [22]. 99mTc-BAT-AV-45 displayed suitable lipophilicity with the value of log P(2.07 ± 0.087) and the results are listed in Table 1.
Electrophoresis
In electrophoresis experiments the applied radioactivity of 99mTc-BAT-AV-45 remained at the spotted point, which indicates that the compounds is uncharged at physiological pH.
Conclusions
In conclusion, we successfully designed and synthesized novel styrylpyridine derivative conjugated with 99mTc. Satisfactory results were obtained by optimizing the radiolabeling conditions. 99mTc-BAT-AV-45 was prepared at an average yield of 95 ± 1% by mixing 50 μg of BAT-AV-45, 10 mg of sodium glucoheptonate, and stannous chloride (50 μg) dissolved in HCl (0.1 N) at pH value (5–6) at 90 °C for 30 min. 99mTc-BAT-AV-45 with suitable lipophilicity (log P = 2.07 ± 0.087) was stable and uncharged at physiological pH. Further studies are needed to apply 99mTc-BAT-AV-45 for Aβ plaques imaging.
References
Selkoe DJ (2000) The origins of Alzheimer disease. JAMA 283(12):1615–1617
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356
Jurisson SS, Lydon JD (1999) Potential technetium small molecule radiopharmaceuticals. Chem Rev 99:2205
Meegalla SK, Plössl K, Kung MP et al (1997) Synthesis and characterization of technetium -99m-labeled tropanes as dopamine transporter-imaging agents. J Med Chem. 40(1):9–17
Kung HF, Kim HJ, Kung MP et al (1996) Imaging of dopamine transporters in humans with technetium-99m TRODAT-1. Eur J Nucl Med 23(11):1527–1530
Mozley PD, Schneider JS, Acton PD et al (2000) Binding of [99mTc]TRODAT-1 to dopamine transporters in patients with Parkinson’s disease and in healthy volunteers. J Nucl Med 41(4):584–589
Ono M, Saji H (2011) SPECT imaging agents for detecting cerebral β-amyloid plaques. Int J Mol Imaging 13:543267
Cui M, Tang R, Li Z, Ren H, Liu B (2011) 99mTc- and Re-labeled 6-dialkylamino -2-naphthyl- ethylidene derivatives as imaging probes for β-amyloid plaques. Bioorg Med Chem Lett. 21(3):1064–1068
Ono M, Ikeoka R, Watanabe H et al (2010) 99mTc/Re complexes based on flavone and aurone as SPECT probes for imaging cerebral β-amyloid plaques. Bioorg Med Chem Lett. 20(19):5743–5748
Yang Y, Zhu L, Cui M, Tang R, Zhang H (2010) Preparation of classical Re/99mTc (CO)3(+) and novel 99mTc(CO)2(NO)2+ cores complexed with flavonol derivatives and their binding characteristics for A beta(1–40) aggregates. Bioorg Med Chem Lett. 20(17):5337–5344
Zhuang Z-P, Kung M-P, Ono M et al (2003) Syntheses of two potential ligands for Tc-99m labeling as diagnosis agents of Alzheimer’s disease. Chin J Chem 21(7):824–832
Lin KS, Debnath ML, Mathis CA, Klunk WE (2009) Synthesis and beta-amyloid binding properties of rhenium 2-phenylbenzothiazoles. Bioorg Med Chem Lett 19(8):2258–2262
Serdons K, Van der Ghinste D, Van Eeckhoudt M et al (2009) Synthesis and evaluation of two uncharged 99mTc-labeled derivatives of thioflavin-T as potential tracer agents for fibrillar brain amyloid. J Labelled Comp Rad 52(6):227–235
Chen X, Yu P, Zhang L, Liu B (2008) Synthesis and biological evaluation of 99mTc, Re-monoamine-monoamide conjugated to 2-(4-aminophenyl)benzothiazole as potential probes for β-amyloid plaques in the brain. Bioorg Med Chem Lett 18:1442–1445
Serdons K, Verduyckt T, Cleynhens J et al (2007) Synthesis and evaluation of a 99mTc- BAT-phenylbenzothiazole conjugate as a potential in vivo tracer for visualization of amyloid β. Bioorg Med Chem Lett 17:6086–6090
Choi SR, Golding G, Zhuang Z et al (2009) Preclinical properties of 18F-AV-45: a PET agent for Abeta plaques in the brain. J Nucl Med. 50(11):1887–1894
Wong DF, Rosenberg PB, Zhou Y et al (2010) In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F 18). J Nucl Med 51(6):913–920
Lin KJ, Hsu WC, Hsiao IT et al (2010) Whole-body biodistribution and brain PET imaging with [18F]AV-45, a novel amyloid imaging agent—a pilot study. Nucl Med Biol 37(4):497–508
Oya S, Plossl K, Kung MP (1998) Small and neutral Tc(v)O BAT, bisaminoethanethiol (N2S2) complexes for developing new brain imaging agents. Nucl Med Biol 25:135–140
US 2010/0172836 A1
Choi SR, Kung MP, Plössl K, Meegalla S, Kung HF (1999) An improved kit formulation of a dopamine transporter imaging agent: [Tc-99m]TRODAT-1. Nucl Med Biol 26(4):461–466
Dishino DD, Welch MJ, Kilbourn MR, Raichle ME (1983) Relationship between lipophilicity and brain extraction of C-11-labeled radiopharmaceuticals. J Nucl Med 24:1030–1038
Acknowledgments
This work was supported by the Jiangsu Natural Science Foundation(No. BK2011167 and No. BK 2010157).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Zhou, X. & Qin, X. Preparation, quality control and physico-chemical properties of 99mTc-BAT-AV-45. J Radioanal Nucl Chem 292, 1377–1383 (2012). https://doi.org/10.1007/s10967-012-1657-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-1657-6