The use of tin(II) chloride in amount above its required mole ratio as reducing agent can lead to instability of peptide-based radiopharmaceuticals. In this work, we optimize the radiolabeling conditions, especially tin(II) chloride amount for 99mTc-labeling of one 6-hydrazinonicotinamide (HYNIC) conjugated peptide in the presence of tricine and nicotinic acid (NA). Implementation of this procedure leads to 99mTc-labeling of this HYNIC-conjugated peptide in high radiochemical yield (>95%). By comparison, our experimental results can provide an efficacious strategy for 99mTc-labeling of HYNIC-conjugated biomolecules in the presence of tricine/NA. The resulting 99mTc-labeled peptide, without the need for any chemical modification and pretreatment, shows good stability in saline and human serum. This work can provide a new perspective for 99mTc-labeling of HYNIC-conjugated biomolecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. INTRODUCTION
Today, peptide-based radiopharmaceuticals are the spotlight of comprehensive research area for tumor diagnostics and therapy because of their surprising traits and eye-catching nature [1,2, – 3]. Peptides are small biomolecules that penetrate rapidly in target tissues and clear easily from non-target tissues. Peptides have low toxicity and show little immunogenicity [4, 5]. Recent literature survey shows that peptide- based radiopharmaceuticals have been introduced into clinical work more often than in the past [6]. Despite fantastic raising of positron emission tomography (PET) and availability of radionuclides, peptide-based radiopharmaceuticals are mostly labeled with technetium-99m (99m Tc). Technetium- 99m is the most commonly used radioisotope in nuclear medicine [7, 8]. It can also be claimed that 6-hydrazinonicotinamide (HYNIC) is the ideal bifunctional coupling agent (BFCA) for 99m Tc-labeling of biomolecules, because of its rapid and efficient labeling capability [9, 10]. In addition, specific tumor uptake and appropriate pharmacokinetics have been achieved by modifying various co-ligands [11].
According to previously published data, in most conventional 99mTc-labeling method of HYNIC-conjugated biomolecules, the used mole ratio of tin(II) chloride is significantly higher than the mole ratio of biomolecules. However, the additional amount of tin(II) chloride can lead to radiopharmaceutical instability [12, 13]. Furthermore, 99mTc-labeled biomolecules have been obtained by using some pretreatment and chemical modification such as addition of ex situ buffers [14, 15]. To the best of our knowledge, there have been no reports on the optimized (minimized) amount of tin(II) chloride for 99mTc-labeling of HYNIC-conjugated biomolecules in the presence of tricine and nicotinic acid (NA) to date. Therefore, optimized and simplified 99mTc-labeling procedure of HYNIC-conjugated peptides in the presence of tricine/NA can be of great interest. In this research article, we introduce optimized conditions, particularly in respect of the amount of tin(II) chloride and consequently simplified 99mTc-labeling strategy for obtaining one HYNIC-conjugated peptide in the presence of tricine/NA that can be appropriate for any HYNIC-conjugated biomolecules.
2. EXPERIMENTAL
2.1. Reagents and Instrumentation
The HYNIC-conjugated peptide KRWrNM (M-6) (Fig. 1), with a purity of >95% was purchased from PepmicCo Ltd. (China). Sodium pertechnetate was eluted from 99Mo/99mTc radionuclide generator (Pars Isotope, Tehran, Iran). All reagents were made using analytical grade chemicals (Sigma-Aldrich, Munich, Germany) and doubly deionized (dd) water through standard protocols. After labeling, the radiochemical yield was evaluated through aluminum supported thin-layer chromatography (TLC) on silica gel (TLC SG; Merck) strips and confirmed by reverse phase high performance liquid chromatography (RP-HPLC). The distribution of radioactivity on the silica gel strips was scanned using a Lablogic Mini-Scan TLC scanner and analyzed with Lura image analysis software (Sheffield, UK). Analytical RP-HPLC was performed on a Knauer HPLC system (Germany). The HPLC analyses of radiolabeled peptide were performed on Lablogic system equipped with radioactivity gamma and UV detector set at 240 nm, and Eurospher 100–5 C18 (4.6 250 mm) column with precolumn. The gradient eluent consisted of 0.1% TFA in water (solvent A) and 0.1% TFA in acetonitrile (solvent B) in the following gradient program: 0–10 min, 90% solvent A; 10–15 min, 30% solvent A; 15–20 min, 100% solvent B; 20 – 30 min, 100% solvent B for a total time of 25 or 30 min. The flow rate was 1.0 mL/min. All solvents were filtered and degassed before entering the column. Under these HPLC conditions, pertechnetate and 99mTc co-ligands had a retention time of 4–6 min. Radioactivity in the samples was measured using a gamma counter with NaI(Tl) detector (Delshid, Iran).
2.2. 99m Tc-Labelling of HYNIC-Peptide with Tricine/NA
Various parameters influencing the radiochemical yield (RCY) including pH (Table 1), amount of peptide added (Table 2), co-ligand mole ratios (Table 3) and amount of stannous ions added (Table 4) were analyzed. The only parameter having great effect on the RCY was the amount of stannous ions added (Fig. 2 and Table 4). For this purpose, 20 μL of HYNIC-M-6 (1 mg/mL in H2O) was added to a vial containing 15 mg of tricine and 10 mg of NA in 0.25 mL of ddH2O. To this solution was added 1.25 μL (~12.5 nmol) of tin chloride solution (2 mg/mL SnCl2·2H2O in nitrogen-purged 0.1 m HCl). Finally, (5 – 10) mCi of Na99mTcO4 was added to the solution and the vial was heated for 15 min at 95°C in a shielded container. The pH vakue of the reaction mixture was adjusted at 5. After cooling to room temperature, the reaction mixture was analyzed by TLC and HPLC. TLC was performed using three systems: 2- butanone for determination of free 99mTcO4(Rf 1), 0.1 M citric buffer (pH 5) for detection of 99mTcO4 and non-peptide-bound 99mTc-coligands (Rf 1), and C2H5OH:NH4OH:H2O (1:1:1v/v) for detection of 99mTc-colloid (Rf 0). The radiolabeled solution was then subjected to quality control by analytic RP-HPLC using gradient elution as stated in previous section.
2.3. Stability in Solution
The in vitro stability of [99mTc]Tc-M-6 was tested by incubating 100 μL of labeled peptide with 1 mL of the normal saline solution for 1, 4 and 24 h at 25°C. After incubation, [99mTc]Tc-M-6 was assessed by TLC. All experiments were performed in triplicate.
2.4. Protein Binding and Stability in Serum
The percentage of [99mTc]Tc-M-6 bound to plasma proteins and the stability of these in human serum was evaluated by incubating 100 μL of labeled peptide with 500 μL of fresh human serum at 37°C. At 1 and 4 h time points, 100 μL aliquots were treated with 200 μL of ethanol. The samples were centrifuged for 10 min at 4000g to precipitate serum proteins. Supernatants were passed through 0.22 μm filter and then analyzed by RP-HPLC. The total activity of the supernatant and sediment was measured by well-type NaI γ-counter to determine the percentage of [99mTc]Tc-M-6 bound or transferred to serum proteins.
2.5. Calculation of Log P
Aliquot (100 μL) of [99mTc]Tc-M-6 was added to a tube containing 1 mL n-octanol and 1 mL water. The tube was vortexed vigorously for 10 min and then centrifuged for 5 min at 4000g. Three aliquots of 100 μL were sampled from each layer and counted by γ-counter. The partition ratios were calculated by dividing the counts in the organic phase by that in the aqueous phase per unit volume.
3. RESULTS
3.1. Radiolabeling
Radiolabeling of M-6 was performed efficiently with technetium-99m using tricine/NA as co-ligand system. The effects of various parameters on the radiochemical yield (RCY) are shown in tables including pH (Table 1), amount of peptide (Table 2), co-ligand mole ratio (Table 3) and amount of Sn(II) chloride (Table 4). The only parameter having great effect on RCY was the amount of stannous ions added. The effect of Sn(II) chloride amount on the RCY is illustrated in Fig. 2 and Table 4. The labeling yield of [99mTc]Tc-M-6 was >95% (n = 3). The γ-chromatogram displays a single peak with a retention time of 17.05 min for [99mTc]Tc-M-6 (Fig. 3). The percentage of 99mTc-colloid in this radiolabeled sample was detected with TLC scanner (Fig. 4).
3.2. Stability in Solution and Log P
The experimental stability in saline was monitored during 24 h. The lipophilicity of [99mTc]Tc-M-6 was evaluated by partition between n-octanol and water. The log P value of [99mTc]Tc-M-6 was –3.71 ± 0.02 (Table 5).
3.3. Stability in Serum and Protein Binding
The stability of [99mTc]Tc-M-6 in human serum for up to 4 h was examined by RP-HPLC analysis. Figure 5 shows γ-chromatograms of M-6 in human serum measured after 1 h and 4 h. [99m Tc]Tc-M-6 showed stability of approximately >94% over 4 h. The protein binding after 4 h incubation of [99m Tc]Tc-M-6 in serum was 32.82 ± 0.03% (Table 5).
4. DISCUSSION
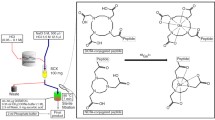
In this work, we have optimized the minimum amount of tin(II) chloride and other conditions for 99mTc-labeling procedure of one HYNIC-conjugated peptide in the presence of tricine/NA. As shown in Fig. 6, the proposed procedure involves dissolving adequate amount of tricine and nicotinic acid as co-ligand system, adequate amount of HYNIC-conjugated peptide as a potential tracer, trace and unique amount of stannous ion as a reducing agent and adequate activity of technetium-99m as a radionuclide, in pure water. In situ heating of the solution for 15 min at 95°C in a shielded container is an essential requirement in this method. In this work, tricine/NA can have a dual role as a co-ligand system and pH stabilizing. In connection with the second role, it should be noted that function of buffer (weak acid) is best when the pKa of the weak acid is close to the desired working pH (pH = pKa) [16,17, – 18]. Thus, nicotinic acid (pKa = 4.85 at 25°C) approximately have a maximum buffer capacity in reaction cocktail (pH 5). Application of this issue can be a key factor for ex situ buffer removing in 99mTc-labeling of HYNIC-conjugated biomolecules.
The most significant advantage of this procedure is to optimize the least amount of tin(II) chloride for 99mTc-labeling of HYNIC-conjugated peptide in the presence of Tricine/NA. This optimization of tin(II) chloride can be helpful because it can increase the stability of HYNIC-peptide solution without having impressive effect on the polarity or pharmacokinetics of HYNIC-peptide [12, 13]. According to Fig. 2 and Table 4, when the amount of SnCl2 was changed from 2.5 to 15 μg (i.e., peptide:Sn2+mole ratio changed from 2:1 to 1:3), the 99m-technetium in colloid increased, resulting in less RCY.
Generally, Table 6 summarizes the proposed 99mTc-labeling conditions in comparison to other conventional 99mTc-labeling methods of HYNIC-conjugated peptides in the presence of tricine/NA [10, 19,20, – 21]. As can be seen from Table 6, the prominent features of these conditions are the 99mTc-labeling of peptide without conventional (high) amount of SnCl2, ex situ buffer and pretreatment, in pure water (ddH2O).
The radiochemical yield of labeled HYNIC-conjugated peptide was calculated by RP-HPLC and TLC. Fig. 3 and Fig. 4 show RP-HPLC and TLC γ-chromatogram of [99mTc]Tc-M-6 in the presence of Tricine/NA, respectively. Accordingly, the first peak (A) correspond to 99mTcO4 and 99mTc-coligands formation at the retention time of 4–6 min and the second peak (B) correspond to [99mTc]Tc-M-6 formation at a retention time of 17.05 min in Fig. 3. In Fig. 4, the first peak (A) corresponds to 99mTc-colloid formation and the second peak (B) corresponds to [99mTc]Tc-M-6 formation. The %RCY was calculated by the following equation:
Subsequently, the stability of [99mTc]Tc-M-6 in saline and human serum was examined up to 24 and 4 h by TLC and RP-HPLC analysis, respectively. As can be seen from data in Table 5 and Fig. 5, [99mTc]Tc-M-6 is stable in saline and human serum (approximately >94%) for up to 24 and 4 h, respectively. Thus, [99mTc]Tc-M-6, shows good stability in both saline and human serum. We profoundly believe that the use of this strategy, along with consideration of the above-mentioned advantages, can make a new challenging outlook and good foundation for the 99mTc-labeling of many HYNIC-conjugated biomolecules.
FUNDING
This work was a subject part of the thesis of Sajad Kaihani as a PhD student of the Mazandaran University of Medical Sciences and was supported with Grant Number 6301.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
References
P. Laverman, W. J. McBride, R. M. Sharkey, et al., Comp. Radiopharm., 57(4), 219 – 223 (2014).
C. Dong and Z. Liu, Curr. Med. Chem., 21(1), 139 – 152 (2014).
S. M. Okarvi, Med. Res. Rev., 24(3), 357 – 397 (2004).
I. Dijkgraaf, O. C. Boerman, W. J. Oyen, et al., Anti-Cancer Agents Med. Chem., 7(5), 543 – 551 (2007).
F. Rezazadeh and N. Sadeghzadeh, Chem. Biol. Drug. Des., 93(3), 205 – 221 (2019).
M. Gotthardt, O. C. Boermann, T. M. Behr, et al., Curr. Pharm. Des., 10(24), 2951 – 2963 (2004).
K. Ju, J. Lee, H. ur Rehman, et al., Nucl. Eng. Technol., 51(1), 176 – 189 (2019).
W. C. Eckelman, M. Bonardi, and W. A. Volkert, Nucl. Med. Biol., 35(5), 523 – 527 (2008).
D. Shamshirian, M. Erfani, D. Beiki, et al., Iran. J. Pharm. Res: IJPR., 15(3), 349 – 360 (2016).
R. C. King, M. B.-U. Surfraz, S. C. Biagini, et al., Dalton. Trans., 43, 4998 – 5007 (2007).
L. K. Meszaros, A. Dose, S. C. Biagini, et al., Inorg. Chim. Acta, 363(6), 1059 – 1069 (2010).
S. Srivastava, G. Meinken, T. Smith, et al., Int. J. Appl. Radiat. Isot., 28(1 – 2), 83 – 95 (1977).
S. Chakraborty, S. Das, R. Chakravarty, et al., J. Labelled Comp. Radiopharm., 62(12), 823 – 834 (2019).
S. Kaihani and N. Sadeghzadeh, J. Labelled Comp. Radiopharm., 63(14), 582 – 596 (2020).
C. Decristoforo and S. J. Mather, Nucl. Med. Biol., 26(4), 389 – 396 (1999).
C. Mohan, Buffers. A guide for the preparation and use of buffers in biological systems, Calbiochem (2003), pp. 20 – 21.
D. D. Perrin, Buffers for pH and Metal Ion Control, Springer Science & Business Media (2012).
D. M. Bers, C. W. Patton, and R. Nuccitelli, Methods Cell. Biol., 99, 1 – 26 (2010).
C. Decristoforo, L. Melendez-Alafort, J. Sosabowski, et al., J. Nucl. Med., 41(6), 1114 – 1119 (2000).
C. Decristoforo, B. Faintuch-Linkowski, A. Rey, et al., Nucl. Med. Biol., 33(8), 945 – 952 (2006).
P. Laverman, M. Béhé,W. J. Oyen, et al., Bioconjug. Chem., 15, 561 – 568 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaihani, S., Sadeghzadeh, N. Effect of Tin(II) Chloride Amount on 99mTc-Labeling of One 6-Hydrazinonicotinamide-Conjugated Peptide in the Presence of Tricine/Nicotinic Acid. Pharm Chem J 56, 1075–1081 (2022). https://doi.org/10.1007/s11094-022-02756-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-022-02756-2