Abstract
The ammonium citrate tribasic was successfully modified to attapulgite clay and the effect of modifying was characterized by FTIR and XRD techniques. Experimental results showed that the ammonium citrate tribasic modified attapulgite clay had a strong sorption ability to remove Th(IV) from aqueous solutions. The sorption of Th(IV) from aqueous solutions has been systematically investigated as a function of several variables including contact time, solid content, pH, ionic strength, Fulvic acid (FA)/humic acid (HA) and temperature under ambient conditions. The results indicate that the sorption of Th(IV) onto ammonium citrate tribasic modified attapulgite clay is strongly dependent on pH, Th(IV) initial concentration, ionic strength, temperature and HA/FA. Surface complexation and ionic exchange are the main sorption mechanisms. Sorption of Th(IV) onto ammonium citrate tribasic modified attapulgite is quick and can be fitted by a pseudo-second-order rate model very well. Sorption of Th(IV) onto ammonium citrate tribasic modified attapulgite is promoted at higher temperature and the sorption reaction is an endothermic process. Langmuir isotherm model fits the experimental data better than Freundlich and D-R isotherm models. The results suggest that the ammonium citrate tribasic modified attapulgite sample is a suitable material in the preconcentration and solidification of radionuclide Th(IV) from large volumes of aqueous solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The disposal of high-level radioactive waste is one of the major problems in the world today because of its long half life and possible transport of into the environment. In the last decade, the removal of radionuclide ions from aqueous solution has been studied extensively [1–8]. Thorium is only stable at its valence +IV state in solution, and is usually used as a chemical analogue of tetravalent radionuclides as Zr, Hf, Np, U and Pu, which are difficult to study and to keep in the tetravalent form [9]. The fate of Th(IV) in clay minerals and in the environment is controlled by sorption, desorption, diffusion, migration and complexation on all kinds of clay minerals and oxides. In recent years, sorption of Th(IV) on different sorbents has been a subject of a number of theoretical studies. Sheng et al. [10] studied the sorption properties of Th(IV) on raw diatomite and the result indicated that sorption of Th(IV) is strongly dependent on ionic strength at pH < 3, and is independent of ionic strength at pH > 3. Outer-sphere complexation or ion exchange may be the main sorption mechanism of Th(IV) to diatomite at low pH values.
Attapulgite has attracted great interest in nuclear waste management because of its outstanding properties [11]. Attapulgite is a hydrated octahedral layered magnesium aluminum silicate present in the natural environment as a fabrillar silicate clay mineral. It has permanent negative charges on its surface, which enable it to be modified by cationic surfactants, to enhance contaminant retention and to retard contaminant migration. In addition, some isomorphic substitutions in the tetrahedral layer, such as Al3+ for Si4+, develop negatively charged adsorption sites able to electro-statically adsorb cations. In last decades, attapulgite has been studied intensively [12, 13], However, the study of the sorption of Th(IV) onto attapulgite is still scarce.
The aim of the present investigation is to study the sorption mechanism of Th(IV) ions onto the organomodified attapulgite and to understand the way Th(IV) ions interact with organomodified attapulgite. Towards this aim, the effect of various parameters such as contact time, solid content, pH, ionic strength, Fulvic acid (FA)/humic acid (HA) and temperature on the sorption process have been investigated. Thermodynamic parameters of Th(IV) sorption isotherms with the Langmuir, Freundlich and D-R models have been calculated to interpret the results.
Experimental
Materials
Chemical reagents NaNO3, NaOH, HNO3 and C6H8O7·H2O used in the experiments were purchased in analytic purity and used without any purification. All the solutions were prepared with Milli-Q water under ambient conditions. No attempts were made to exclude air in the preparation of the solutions. The purified attapulgite used in this study is a product of Jiuchuan company operating in Xuyi county (Jiangsu, China), and was converted into organomodified attapulgite by ammonium citrate tribasic. HA and FA were extracted from the soil of Gansu province (China), and were characterized in detail [14, 15].
Preparation of organomodified attapulgite
The purified attapulgite and ammonium citrate tribasic were mixed at a mass ratio of 1:2.4 in water and adjusted the volume to 100 mL. After stirred for 4 h (pH = 7.0 ± 0.1), the mixture was transferred to a bottle and refluxed for 50 h. Then the sample was filtered out, rinsed with Milli-Q water until the neutral pH and dried at 105 °C overnight. The powder was grounded and passed 200 meshes to get organomodified attapulgite. The purified attapulgite and organomodified attapulgite samples were characterized by FTIR and XRD.
Experimental sorption procedure
The experiments were carried out under ambient conditions at T = 25 ± 1 °C by using batch techniques. All solutions were prepared with Milli-Q water, and all experiments were conducted in polyethylene tubes. The aqueous suspension was mixed with a solution containing the background electrolyte NaNO3, HA, FA, organomodified attapulgite and Milli-Q water. HA or FA was first equilibrated with the organomodified attapulgite suspension for 2 days, and then Th(IV) solution was added into the HA/FA coated attapulgite suspension to study the sorption of Th(IV) on HA/FA coated organomodified attapulgite. The pH value of the solution was adjusted with negligible amounts of 0.1 mol/L NaOH or HNO3 solution. The polyethylene tubes containing suspension were shaken for 24 h to attain sorption equilibration, and then the solid was separated from supernatant solution by centrifugation at 9,000 rpm for 20 min.
The concentration of Th(IV) was determined by spectrophotomery at 650 nm by using the Th-arsenazo(III) complex. The amount of Th(IV) adsorbed on organomodified attapulgite was calculated from the difference between the initial and the equilibrium concentration. All experimental data were the averages of duplicate or triplicate experiments. The relative errors of the data were about 5%.
Experimental data analysis
The sorption of Th(IV) was expressed in terms of distribution coefficient (Kd) and sorption percentage (%), which were derived from the following equations:
The amount of Th(IV) ion adsorbed on the solid is calculated as follows:
where C 0 (mol/L) is the initial concentration of Th(IV) in suspension; C eq (mol/L) is the equilibrium one in supernatant after centrifugation; V (mL) is the volume of the suspension and m (g) is the mass of solid; q (mol/g) is the amount of metal ions adsorbed on per weight unit of solid after equilibrium.
According to the works of Ho and Mckay [16, 17], pseudo-first-order equation is generally expressed as:
Eq. 4 is integrated for the boundary conditions t = 0 to t > 0 (q t = 0 to q t > 0) and then its linear form can be formulated as:
The pseudo-second-order shows how the rate dependent on the sorption capacity and describes the kinetics of sorption as follows [16, 17]:
Integrating Eq. 6 for the boundary conditions t = 0 to t > 0 (q t = 0 to q t > 0), and rearranging to obtain the linear form as:
the linear fit of t/q t to t can achieve the sorption capacity at equilibrium (q e) and the rate constant of pseudo-second-order sorption (k 2).
The possibility of intraparticle diffusion (k i) is explored by using the following equation [18]:
plots of q versus t 1/2 for different initial concentrations can achieve the value of k i from the slope of the regression line.
The Langmuir, Freundlich and Dubinin-Radushkevich (D-R) isotherm models are used most commonly to describe the sorption characteristic of sorbent. The Langmuir isotherm [19] is valid for monolayer sorption to surface:
where q max (mol/g) and b (L/mg) are Langmuir constants related to sorption capacity and sorption energy, respectively.
The Freundlich isotherm model [19] can illustrate properly the sorption data at low and intermediate concentrations on heterogeneous surfaces. The model has the following form:
both K F (mol1−n Ln g−1) and n are empirical constants, being indicative of the extent of sorption and the degree of nonlinearity between solution and concentration, respectively.
The D-R isotherm is more general than the Langmuir, because it does not assume a homogeneous surface or constant sorption potential. The D-R equation is expressed as [20]:
Eq. 11 can be expressed in linear form:
where q and q max are defined above, β is the activity coefficient related to the mean sorption energy (mol2/kJ2); ε is the Polanyi potential; R is the ideal gas constant (8.2145 J/mol/K) and T is the absolute temperature in Kelvin (K). E (kJ/mol) is defined as the free energy change required to transfer 1 mol of ions from solution to the solid surface, which equals to:
the magnitude of E is useful for estimating the type of sorption reaction. If E is in the range of 8–16 kJ/mol, the sorption process is governed by chemical ionexchange. In the case of E < 8 kJ/mol, physical forces may affect the sorption. On the other hand, the sorption is governed by particle diffusion if E > 16 kJ/mol [21, 22].
Results and discussion
Characterization of organomodified attapulgite
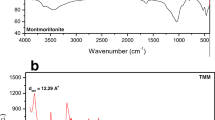
The FTIR spectra of pure attapulgite sample and organomodified attapulgite sample by ammonium citrate tribasic are shown in Fig. 1. According to the FTIR spectra of the two samples, the peaks at 3620, 3560 and 3423 cm−1 are corresponding to the stretching vibration of Al–OH unite, Fe–OH unite and zeolitic water, respectively. The peaks at 1,018 and 474 cm−1 are attributed to Si–O–Si bonds, the peak at 800 cm−1 may be corresponds to the stretching vibration of Al–O–Si. Comparing the pure attapulgite sample and organomodified attapulgite sample, the intensity of peaks at 1018, 474 and 800 cm−1 decreases obviously in organomodified attapulgite sample, it indicates that the structures of Si–O–Si and Al–O–Si have been destroyed partly that may be contributed to reaction between ammonium citrate tribasic and attapulgite. In addition, it is necessary to notice that two new peaks appears at 2,917 and 2,973 cm−1 in organomodified attapulgite sample which correspond to asymmetrical stretching vibration and symmetrical stretching vibration of – CH2 unite. The peaks at 3,620 and 3,560 cm−1 in attapulgite sample have disappeared in organomodified attapulgite sample. It is also well known that the peak at 1,658 cm−1 in attapulgite sample corresponds to the bend vibration of zeolitic water has shifted to 1,630 cm−1 in organomodified attapulgite sample, it may be attributed to the asymmetric stretching mode of carbonyl groups (C=O) because of the substitute of zeolitic water by ammonium citrate tribasic molecules. All these results indicate that ammonium citrate tribasic molecules have been successfully grafted to attapulgite via reaction with the structure hydroxyl or zeolitic water.
Figure 2 shows the XRD analysis of pure attapulgite and organomodified attapulgite samples. The peak positions 2θ = 8.4o, 19.7o, 27.5o, 34.6o and 42.5o are exactly the same in pure attapulgite and organomodified attapulgite samples, which indicates the attapulgite crystal does not change after ammonium citrate tribasic molecules are successfully grafted on attapulgite. The peak positions 2θ = 13.6o and 16.4o correspond to the Si–O–Si crystalline layer disappeared in organomodified attapulgite sample which also indicates that ammonium citrate tribasic molecules have been grafted on attapulgite successfully.
Effect of contact time
Sorption of Th(IV) from aqueous solutions to organomodified attapulgite by ammonium citrate tribasic as a function of contact time at pH 2.00 ± 0.01 and in 0.01 mol/L NaNO3 solutions are shown in Fig. 3. The sorption of Th(IV) increases with increasing contact time. The removal percentage of Th(IV) is strongly dependent on the initial concentrations of Th(IV). For the initial Th(IV) concentrations of 5 and 10 mg/L, the sorption of Th(IV) to organomodified attapulgite mainly occurs in the first contact time of 5 h. The fast sorption indicates that strong chemical sorption or strong surface complexation rather than physical sorption contributes to sorption velocity of Th(IV) on organomodified attapulgite. The result is consistent with the sorption of Th(IV) onto Al-pillared rectorite studied by Yu et al. [23]. For the initial Th(IV) concentration of 15.0 mg/L, the sorption of Th(IV) from aqueous solution to organomodified attapulgite increases with increasing contact time too.
The kinetic sorption data were simulated with pseudo-first-order-model, Pseudo-second-order model and intra-particle diffusion model, respectively. The results are listed in Table 1. From the values of R 2, the kinetic sorption of Th(IV) can be fitted by pseudo-first-order-model and Pseudo-second-order model but not can be fitted by intra-particle diffusion model. However, pseudo-second-order model fits better than the pseudo-first-order-model. The results indicated that the organomodified attapulgite is an efficient material in the removal of Th(IV) from aqueous solution at low pH values.
Effect of solid content
The solid content is an important parameter because this determines the capacity of an adsorbent for a given initial concentration of the adsorbate. Sorption of Th(IV) as a function of sorbent content is shown in Fig. 4. One can see that the sorption percentage of Th(IV) increases with increasing attapulgite content in the suspension. The function sites on attapulgite surfaces increases with increasing of attapulgite content, thereby the sorption percentage of Th(IV) increases reasonably.
It is necessary to note that the distribution coefficient (K d) of Th(IV) on organomodified attapulgite does not change with the increasing solid content within the experimental uncertainty, which is consistent with the physicochemical properties of K d. At low concentration of Th(IV) and low content of attapulgite, the K d value is independent of solid content. The result is consistent with the sorption of Cu(II) onto GMZ bentonite [24] and the sorption of Ni(II) onto NKF-6 zeolite [25].
Effect of pH and ionic strength
The pH of the aqueous solution is an important variable that controls cationic sorption onto clay surface. This is due to the change of clay surface properties and the Th(IV) species with an increase of pH. Sorption of Th(IV) on organomodified attapulgite as a function of pH in 0.001, 0.01 and 0.1 mol/L NaNO3 solutions are shown in Fig. 5. As can be seen from Fig. 5, the Th(IV) sorption increases quickly between 1 and 4, attains a maximum value around 4.0 and does not change considerably for higher pH values. It is well known that the species of Th(IV) are strongly dependent on pH values and the species of Th(IV) are crucial to Th(IV) sorption. The relative distribution of Th(IV) species in the aqueous solution calculated with the thermodynamic constants is shown in Fig. 6. One can see clearly from Fig. 6 that Th(IV) can easily form precipitation at pH > 4 because of the low solubility of Th(OH)4 (Ksp = 2.0 × 10−45) [10]. The precipitation curve of Th(IV) at the concentration of 10 mg/L is also shown in Fig. 5. It is clear that Th(IV) starts to form precipitation at about pH 4 if Th(IV) is not adsorbed on attapulgite. At pH < 4, the sorption percent of Th(IV) reaches to about 100%, so the removal of Th(IV) from solution to attapulgite is not attributed to the precipitation. The strong sorption of Th(IV) may be attributed to surface complexation or strong chemical sorption. In aqueous solutions the surface groups of attapulgite can be protolyzed in acid media \( \left( { \equiv SOH + H^{ + } \leftrightarrow \equiv SOH_{2}^{ + } } \right) \) and alkaline media \( \left( { \equiv SOH \leftrightarrow \equiv SO^{ - } + H^{ + } } \right) \). Therefore the concentrations of surface (≡SOH, ≡SOH 2 +, ≡SO −) of attapulgite change at different pH values. The sorption mechanisms can be described as follows:
With increasing pH, the number of negatively charged ≡SO− groups increases and the hydrolysis of Th4+ also increases. The strong pH dependent sorption indicates that the sorption is dominated by surface complexation.
One can also see clearly that the sorption of Th(IV) decreases with increasing ionic strength, the ionic strength dependent sorption indicates that cation exchange contributes to the sorption. The results are consistent with the results of Ni(II) sorption on Na-attapulgite [26], bentonite [27] and goethite [28].
To illustrate the variation and relationship of pH, C eq and q, we plot experimental data of Th(IV) sorption in 0.001, 0.01 and 0.1 mol/L NaNO3 solutions as 3-D plots of pH, C eq and q in Fig. 7. On the pH-q plane, the lines are very similar to that of pH-sorption percent in Fig. 5. On the pH-C eq plane, the projection on the pH-C eq plane is just the inverted image of the projection on the pH-q plane. On the C eq-q plane, the projection is a straight line containing all experimental data. The slope and the intercept calculated from the Ceq-q line are −3.33 and 1.00 × 10−4, which are quite in agreement with the values of m/V = 0.3 g/L and Co·V/m = 1.43 × 10−4 mol/g. Thus the complexity of the sorption edge relative to sorption isotherm is demonstrated. The 3-D plots show the relationship of pH, C eq and q very clearly.
Effect of HA/FA
The pH dependent of Th(IV) sorption onto organomodified attapulgite in the absence and presence of HA/FA was shown in Fig. 8. As can be seen from Fig. 8, a positive effect of HA/FA on the sorption of Th(IV) on attapulgite is observed at pH < 4, while at pH > 4 there is no drastic difference in the sorption percent of Th(IV) on bare and FA/HA coated attapulgite under the experimental analysis. HA/FA has a macromolecular structure, only a small fraction of the “adsorbed” groups is free to interact with metal ions [29, 30]. The complexation between Th(IV) and HA/FA is stronger than that between Th(IV) and attapulgite. The free energy of the formation of HA/FA–Th(IV) complex is smaller than that of attapulgite–Th(IV). At low pH values, the negative charged HA/FA can be easily adsorbed, so the strong complexation ability of surface adsorbed HA/FA with Th(IV) should result in the sorption of Th(IV) on attapulgite surface increasing at pH < 4. At pH > 4, the negatively charged HA/FA is weakly adsorbed on the surface of organomodified attapulgite which is also negative charged as pH increases [31], the HA/FA in solution form soluble complex of HA/FA–Th(IV) and thereby there is no drastic difference in the sorption percent of Th(IV) on bare, FA/HA coated attapulgite at pH > 4.
Sorption isotherms
The sorption of Th(IV) onto organomodified attapulgite at different temperatures of 298.15, 318.15 and 338.15 K, respectively, are shown in Fig. 9A. One can see that the sorption isotherm of Th(IV) onto organomodified attapulgite at temperature of 338.15 K is higher than that of 298.15 and 318.15 K, which indicates that the sorption of Th(IV) onto organomodified attapulgite is promoted at higher temperature and the sorption reaction is an endothermic process [32, 33]. Langmuir, Freundlich and D-R isotherm models are conducted to simulate the sorption data of Th(IV) onto organomodified attapulgite (Fig. 9 b, c, d). The relative parameters are listed in Table 2. It is clear that the Langmuir isotherm model fits the experimental data better than Freundlich and D-R isotherm models, which can be concluded from R 2 values. From the results of D-R model simulation, the E values calculated from the experimental data are 10.53, 10.59 and 12.68 kJ/mol which are in the range of 8–16 kJ/mol, indicating that the sorption is governed by chemical ion-exchange according to D-R model [21, 22]. This suggested that Th(IV) sorption onto organomodified attapulgite should be attributed to chemical sorption rather than physical sorption.
Conclusions
From the results of this paper, one can draw the following conclusions:
-
(1)
Sorption of Th(IV) onto organomodified attapulgite is quick and can be fitted by a pseudo-second-order rate model very well;
-
(2)
Sorption of Th(IV) is strongly dependent on pH values, Th(IV) initial concentration and ionic strength. Surface complexation and ionic exchange contribute to the sorption of Th(IV) onto organomodified attapulgite.
-
(3)
A positive effect of HA/FA on the sorption of Th(IV) on organomodified attapulgite is observed at pH < 4, while at pH > 4 there is no drastic difference in the sorption percent of Th(IV) on bare, FA/HA coated organomodified attapulgite under the experimental analysis.
-
(4)
Sorption of Th(IV) onto organomodified attapulgite is promoted at higher temperature and the sorption reaction is an endothermic process. Langmuir isotherm model fits the experimental data better than Freundlich and D-R isotherm models.
-
(5)
The high sorption ability of organomodified attapulgite and the fast sorption of Th(IV) on attapulgite make attapulgite a suitable candidate in the remove of Th(IV) from large volume solutions.
References
Zhang LP, Zhang H, Ge ZW, Yu XJ (2011) J Radioanal Nucl Chem 288:537–546
Chen L, Yu XJ, Zhao ZD, Pan JS (2008) J Radioanal Nucl Chem 275:209–216
Yu S, Ren A, Cheng J, Song XP, Chen C, Wang X (2007) J Radioanal Nucl Chem 273:129–133
EI-Khouly SH (2006) J Radioanal Nucl Chem 270:391–398
Davila-Rangel JI, Solache-Rios M (2006) J Radioanal Nucl Chem 270:465–471
Khan SA (2003) J Radioanal Nucl Chem 258:3–6
Koudsi UY, Dyer A (2001) J Radioanal Nucl Chem 247:209–219
Hanzel R, Rajec P (2000) J Radioanal Nucl Chem 246:607–615
Choppin GR (1999) Radiochim Acta 85:89–96
Sheng GD, Hu J, Wang XK (2008) Appl Radiat Isot 66:1313–1320
Tan LQ, Jin YL, Chen J, Wu J, Cheng XC, Feng LD (2011) J Radioanal Nucl Chem 289:601–610
Song XP, Wang SW, Chen L, Zhang ML, Dong YH (2009) Appl Radiat Isot 67:1007–1012
Tan XL, Hu J, Zhou X, Yu SM, Wang XK (2008) Radiochim Acta 96:487–495
Tan XL, Wang XK, Geckeis H, Rabung T (2008) Environ Sci Technol 42(17):6532–6537
Tan XL, Fang M, Li JX, Lu Y, Wang XK (2009) J Hazard Mater 168:458–465
Ho YS, McKay G (1998) Process Saf Environ Prot 76:183–191
Yang ST, Li JX, Lu Y, Chen YX, Wang XK (2009) Appl Radiat Isot 671:600–1608
Weber WJ, Morris CJ (1963) J Sanit Eng Div Am Soc Civ Eng 89:31–60
Yang ST, Zhao DL, Zhang H, Lu SS, Chen L, Yu XJ (2010) J Hazard Mater 183:632–640
Xu D, Tan XL, Chen CL, Wang XK (2008) Appl Clay Sci 41:37–46
Ozcan A, Oncu EM, Ozcan AS (2006) Colloids Surf A 277:90–97
Zhao GX, Zhang HX, Fan QH, Ren XM, Li JX, Chen YX, Wang XK (2010) J Hazard Mater 173:661–668
Yu SM, Chen CL, Chang PP, Wang TT, Lu SS, Wang XK (2008) Appl Clay Sci 38:219–226
Li JX, Hu J, Sheng GD, Zhao GX, Huang Q (2009) Colloid Surf A 349:195–201
Zhang H, Yu XJ, Chen L, Jing YJ, Ge ZW (2010) J Environ Radioact 101:1061–1069
Fan QH, Shao DD, Lu Y, Wu WS, Wang XK (2009) Chem Eng J 150:188–195
Hu J, Xu D, Chen L, Wang XK (2009) J Radioanal Nucl Chem 279:701–708
Hu BW, Cheng W, Zhang H, Sheng GD (2010) J Radioanal Nucl Chem 285:389–398
Li XL, Chen CL, Chang PP, Yu SM, Wu WS, Wang XK (2009) Desalination 244:283–292
Chen CL, Wang XK, Jiang H, Hu WP (2007) Colloids Surf A 302:121–125
Chen H, Zhao YG, Wang AQ (2007) J Hazard Mater 149:346–354
Ren XM, Wang SW, Yang ST, Li JX (2010) J Radioanal Nucl Chem 283:253–259
Lu SS, Guo ZQ, Zhang CC, Zhang SW (2011) J Radioanal Nucl Chem 287(2):621–628
Acknowledgment
This work is supported by Jiangsu Natural Science Foundation (BK2009664).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, T., Tan, L. Modifying attapulgite clay by ammonium citrate tribasic for the removal of radionuclide Th(IV) from aqueous solution. J Radioanal Nucl Chem 292, 819–827 (2012). https://doi.org/10.1007/s10967-011-1522-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1522-z