Abstract
The sorption of U(VI) from aqueous solution on MX-80 bentonite was studied as a function of contact time, pH, ionic strength, solid contents, humic acid (HA), fulvic acid (FA) and temperature under ambient conditions using batch technique. The results indicate that sorption of U(VI) on MX-80 bentonite is strongly dependent on pH and ionic strength. The removal of U(VI) to MX-80 bentonite is rather quick and the kinetic sorption data is simulated well by a pseudo-second-order rate equation. The presence of HA enhances the sorption of U(VI) on MX-80 bentonite obviously, but the influence of FA on U(VI) sorption is not obvious. The thermodynamic parameters (ΔH 0, ΔS 0, and ΔG 0) for the sorption of U(VI) calculated from temperature dependent sorption suggest that the sorption reaction is endothermic and spontaneous.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Isotopes of actinide elements are of great importance in the geological disposal of radioactive wastes because of their longevity [1, 2]. Uranium is one representative actinide element which has fundamental importance in nuclear fuel cycle, where it starts as a source and ends up as a final waste component. Uranium released into the environment is predominantly in the hexavalent form as mobile, aqueous uranyl ion (\( {\text{UO}}_{2}^{2 + } \)) under standard environmental conditions [1, 3, 4]. Therefore, uranium is a subject of great concern as a potentially hazardous pollutant to the environment.
Sorption of U(VI) from aqueous solution has been studied extensively [1, 5–7], and the results indicated that sorption of U(VI) was mainly dominated by ion exchange and surface complexation. Although the sorption of U(VI) on various oxides and minerals has been studied extensively, investigations on the sorption of U(VI) on MX-80 bentonite are still scarce, especially the influence of soil humic acid (HA) and fulvic acid (FA) on the sorption of U(VI) on MX-80 bentonite.
Bentonite, which has large specific surface area, high ion-exchange capacity, and sorption affinity for organic and inorganic ions, is considered as the main candidate in the decontamination and treatment of heavy metal ions [8, 9], and as the best backfill material in the disposal of radioactive nuclear wastes [10–12]. This paper is an extension of previous studies in our group [9, 11, 13], where the sorption of Co(II), Pb(II) and Th(IV) on MX-80 bentonite were investigated. The main purpose of this work is to investigate the influence of contact time, pH, ionic strength, HA, FA and temperature on the sorption of U(VI) on MX-80 bentonite. Thermodynamic parameters (i.e., ΔH 0, ΔS 0, ΔG 0) are calculated from the temperature dependent sorption isotherms, and the sorption mechanism is also discussed in detail.
Experimental
Materials
The sample of MX-80 bentonite was derived from SUBATECH laboratory (France) as a gift and well characterized [14, 15]. It consists of montmorillonite whose stoichiometric formula is (Si3.96Al0.04)(Al1.52Mg0.26Fe III0.17 )Na0.18Ca0.11O10(OH)2. The main minerals are quartz, feldspar, calcite (CaCO3, (Ca, Fe)CO3), siderite and pyrite. The sample used in the experiments was milled and passed through the 200-mesh screen.
U(VI) stock solution was prepared by dissolving uranyl nitrate hexahydrate (UO2(NO3)2·6H2O) in double distilled water. Soil HA and FA were extracted from the soil of Hua-Jia county (Gansu, China), and had been characterized in detail [16].
All chemicals used in the experiments were purchased in analytical purity and used without any purification. Double distilled water was used in the experiments.
Sorption procedures
All sorption experiments were carried out under ambient conditions by using batch technique. The stock suspension of MX-80 bentonite and NaClO4 were pre-equilibrated for 24 h, then U(VI) stock solution and HA/FA were added in MX-80 bentonite suspension to achieve the desired concentrations of different components. The volume of the solution was 10 mL and the amount of the solid was 0.5 g/L for each experimental data. The pH values of the solution were adjusted by adding negligible volumes of 0.1 or 0.01 M HClO4 or NaOH. After the suspensions were shaken for 24 h, the solid and liquid phases were separated by centrifugation at 18,000 rpm for 20 min at controlled temperature same to that in the experiments. The concentration of U(VI) was analyzed by spectrophotometry at wavelength 650 nm by using U Arsenazo-III complex. The amount of U(VI) sorbed on MX-80 bentonite was calculated from the difference between initial and equilibrium concentrations.
All experimental data were the average of duplicate or triplicate determinations. The relative errors of the data were about 5%.
Results and discussion
Effect of contact time
Sorption of U(VI) on MX-80 bentonite as a function of contact time is shown in Fig. 1. Sorption of U(VI) on MX-80 bentonite is rapid over the first 1 h of contact time, then the sorption remains constant with increasing time. The quick sorption of U(VI) on MX-80 bentonite suggests that it is dominated by chemical sorption/surface complexation rather than physical sorption. The result also suggests that 1 h is enough to achieve the equilibrium of U(VI) sorption. In the following experiments, the shaking time is fixed for 24 h to make sure that equilibrium is achieved.
To analyze the sorption rate of U(VI) on MX-80 bentonite, a pseudo-second-order rate equation is used to simulate the kinetic sorption [17, 18].
where q t (mg g−1) is the amount of U(VI) sorbed on the solid surface at time t (h), and q e (mg g−1) is the equilibrium sorption capacity. K′ (g mg−1 h−1) is the pseudo-second-order rate constant of sorption. The q e and K′ values calculated from the slope and intercept of plot of t/q t vs. t are 37.369 mg g−1 and 0.178 g mg−1 h−1 under our experimental conditions.
Influence of pH and ionic strength
Figure 2 shows the sorption of U(VI) on MX-80 bentonite as a function of pH in 0.001, 0.01 and 0.1 M NaClO4 solutions. The sorption percent (%) of U(VI) is calculated by the following equation:
where C 0 (mol/L) is the initial concentration of U(VI) in suspension, and C eq (mol/L) is the equilibrium concentration of U(VI) in supernatant after centrifugation. The results show that sorption of U(VI) on MX-80 bentonite is strongly dependent on pH values, and also dependent on ionic strength. The strong pH and ionic strength dependent sorption suggests that sorption of U(VI) is dominated by ion exchange and surface complexation [19, 20]. Generally, surface complexation mechanism is pH dependent, whereas ion exchange mechanism is ionic strength dependent [19–21]. At I = 0.01 M NaClO4, one can see that the sorption of U(VI) increases with increasing pH values at pH < 6, reaches the highest sorption at pH 6–7, and then decreases with increasing pH. The sorption of U(VI) is mainly attributed to the functional groups on MX-80 bentonite surfaces. At pH < 5.5, the sorption curves are shifted to the left at low NaClO4 concentration, whereas at pH > 5.5, the sorption percent is the highest in 0.1 M NaClO4 solution and is the lowest in 0.001 M NaClO4 solution. Kowal-Fouchard et al. [22] studied the sorption of U(VI) on Na-montmorillonite, alumina and silica using laser-induced fluorescence spectroscopy (LIFS) and X-ray photoelectron spectroscopy (XPS), and deduced that the sorption of U(VI) on clay and oxides was mainly dominated by ion exchange or outer-sphere complexation at low pH values, and by inner-sphere complexation at high pH values.
To illustrate the variation and relationship of pH, C eq, and q, the experimental data in Fig. 2 are plotted as three dimensional plots of q, C eq and pH (Fig. 3). On the pH-q plane, the sorption curves of U(VI) are quite similar to the results shown in Fig. 2; On the pH-C eq plane, the concentration of U(VI) remained in solution decreases with increasing pH at pH 2–6, and increases with increasing pH at pH > 7. The projections on the pH-C eq plane are just the inverted images of the projections on the pH-q plane; On the C eq–q plane, all the data lies in a straight line. This is due to the same initial concentration of U(VI) (C 0) and same solid content (m/V) for each experimental point. The following equation can describe the relationship of C eq–q:
Eq. 3 can be rearranged as:
where V (mL) is the volume and m (g) is the mass of bentonite. Thereby, the experimental data of C eq–q lies in a straight line with slope (−V/m) and intercept (C 0 V/m). The slope and the intercept calculated from C eq–q line are −2 and 1.67 × 10−4, which are quite in agreement with the values of m/V = 0.5 g/L and C 0 = 8.33 × 10−5 mol/L. The 3-D plots show that all the data of C eq–q lies in a straight line with slope (−V/m) and intercept (C 0∙V/m) at same initial concentration and same solid content [23].
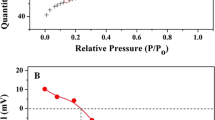
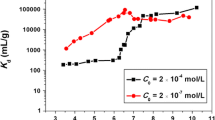
Effect of solid content
Sorption percent (%) and distribution coefficient (K d) of U(VI) on MX-80 bentonite as a function of solid content is shown in Fig. 4. The K d value (mL/g) is calculated using the following equation:
At m/V < 0.9 g/L, the removal of U(VI) from solution increases obviously with increasing solid content. The sorption percent of U(VI) is constant with increasing solid content at m/V > 0.9 g/L. It is well known that the amount of functional groups at MX-80 bentonite surfaces increases with increasing solid content. Thereby, more surface sites are available for the binding of U(VI). As can be seen from Fig. 4, the K d values are constant with increasing solid content, which is consistent with the physicochemical properties of K d values, i.e., the K d value is independent of solid content and solution concentration when both of them are low. The result is very similar with the sorption of Pb(II) on Na-rectorite [19] and sorption of Co(II) and Th(IV) on MX-80 bentonite [11, 13].
Effect of humic acid and fulvic acid
Figures 5 and 6 show pH dependency of U(VI) sorption on MX-80 bentonite in the absence and presence of HA/FA, respectively. The influence of HA on the sorption of U(VI) to HA-bentonite hybrids is much stronger than that of FA at low pH values at the same HA and FA concentrations. At pH < 6.5, the presence of HA enhances U(VI) sorption obviously, whereas FA has little influence on U(VI) sorption. At pH > 6.5, the presence of HA and FA decreases U(VI) sorption with increasing pH. Humic substances (HS) can be considered as a “bridge” to form U-HS-bentonite complexes on bentonite surface at low pH values [16, 20]. The negative charged HA can be easily sorbed on the positive charged MX-80 bentonite surface at low pH values. The strong complexation ability of U(VI) ions with surface sorbed HA results in the increasing sorption of U(VI) on MX-80 bentonite. However, the presence of HA and FA decreases U(VI) sorption on bentonite at high pH values. The surface charge of MX-80 bentonite becomes progressively negative at high pH values. At high pH values, the negative charged FA and HA resist adsorption on the negative surface charged bentonite due to electrostatic repulsion [20]. The fraction of FA or HA remained in solution increases with increasing pH values, and soluble FA/HA-U(VI) complexes are formed in solution and thereby reduce U(VI) sorption at high pH values. At high pH values, as the concentration of HA and FA increase, the sorption percentage of U(VI) on bentonite decrease, which indicates the formation of soluble HA/FA-U(VI) complexes in solution. It may be concluded that the effects of HS on U(VI) sorption to clay are influenced by many parameters such as the nature of the solid particles, the nature of HS and pH [24].
Effect of temperature
Sorption isotherms of U(VI) on MX-80 bentonite at 303, 323, and 333 K are shown in Fig. 7. The sorption isotherm is the highest at T = 333 K and is the lowest at T = 303 K, i.e., sorption of U(VI) on MX-80 bentonite is favored at higher temperatures. This result indicates that the sorption reaction is an endothermic process. In order to get a better understanding of the sorption mechanism, the Langmuir, Freundlich and D–R models are adopted to simulate the sorption isotherms at the three temperatures.
Langmuir sorption isotherm is widely used for modeling the monolayer coverage of sorption surface and assumes that sorption occurs onto a surface containing a finite number of identical sites [25, 26]. The linear form is:
where C eq is the equilibrium concentration of U(VI) in supernatant after centrifugation (mol/L); q is the amount of U(VI) adsorbed on bentonite after equilibrium (mol/g); q max is the maximum amount of U(VI) at complete monolayer coverage (mol/g), and b is a binding constant related to the heat of sorption (L/mol).
Freundlich isotherm model is a semi-empirical equation describing the sorption onto heterogeneity surface [25, 26]:
where k F (L/g) represents the sorption capacity when metal ion equilibrium concentration equals to 1, and n represents the degree of dependence of sorption with equilibrium concentration.
The D–R isotherm is more general than the Langmuir isotherm, because it does not assume a homogeneous surface or constant sorption potential [25, 26]. Its linear form is:
where β is the activity coefficient relates to mean sorption energy (mol2/kJ2), and ε is the Polanyi potential, which is equal to:
where R is the ideal gas constant (8.3145 J mol−1 K−1), and T is the absolute temperature in Kelvin (K).
The sorption isotherms with model simulation are shown in Fig. 8a–c, respectively. The relative parameters calculated from the three models are listed in Table 1. From the correlation coefficients, one can see that the Langmuir model fits the experimental data better than Freundlich and D–R models, which suggests that the sorption of U(VI) on MX-80 bentonite is almost complete monolayer coverage.
Thermodynamic study
The thermodynamic parameters (ΔG 0, ΔS 0 and ΔH 0) for U(VI) sorption on MX-80 bentonite can be determined from the temperature dependence.
The Gibbs free energy change (ΔG 0) is calculated by the following equation [9, 18]:
where K 0 is the sorption equilibrium constant obtaining by plotting ln K d vs. C eq and extrapolating C eq to zero [27].
Standard entropy change (ΔS 0) and average standard enthalpy change (ΔH 0) are calculated by using the following equations [26, 27]:
The values obtained from Eqs. 10–12 are listed in Table 2. The positive value of ΔH 0 indicates that the sorption is an endothermic process. One possible explanation to this positive entropy is that energy is needed to destroy the hydration sheath of U(VI) solved well in water before its sorption onto bentonite. Thereby, the sorption process is favored at higher temperature [18]. The ΔG 0 value accompanied by positive ΔS 0 value is negative as expected for a spontaneous process. The value of ΔG 0 becomes more negative with increasing temperature, indicating more efficient sorption at higher temperature.
Conclusions
From the results of U(VI) sorption on MX-80 bentonite under our experimental conditions, the following conclusions can be drawn:
-
(1)
Sorption of U(VI) on MX-80 bentonite is strongly dependent on pH and ionic strength. The sorption is mainly dominated by surface complexation and ion exchange.
-
(2)
The thermodynamic data calculated from the temperature dependent sorption isotherms indicate that the sorption of U(VI) on MX-80 bentonite is endothermic and spontaneous.
-
(3)
The experimental data of U(VI) sorption on MX-80 bentonite are fitted well by Langmuir model. The sorption of U(VI) on MX-80 bentonite is monolayer coverage.
-
(4)
At low pH values, the presence of HA enhances the sorption of U(VI) on MX-80 bentonite, but the presence of FA does not affect the uptake of U(VI) obviously. At high pH values, the presence of FA/HA reduces the sorption of U(VI) on MX-80 bentonite because of the formation of soluble FA/HA-U(VI) complexes in solution.
-
(5)
All the experimental data of C eq –q lies in a straight line with the slope (−V/m) and intercept (C 0·V/m) if the initial concentration of U(VI) and solid content are same for all the experimental data.
References
Hyun SP, Cho YH, Hahn PS, Kim SJ (2001) J Radioanal Nucl Chem 250:55
Akyil S, Aslani MAA, Eral M (2003) J Radioanal Nucl Chem 256:45
Sylwester ER, Hudson EA, Allen PG (2000) Geochim Cosmochim Acta 64:2431
Liao XP, Lu ZB, Du X, Liu X, Shi B (2004) Environ Sci Technol 38:324
Sachs S, Bernhard G (2008) Chemosphere 72:1441
Baik MH, Cho WJ, Hahn PS (2004) J Radioanal Nucl Chem 260:495
Saho DD, Jiang ZQ, Wang XK, Li JX, Meng YD (2009) J Phys Chem B 113:860
Donat R, Akdogan A, Erdem E, Cetish H (2005) J Colloid Interface Sci 286:43
Xu D, Tan XL, Chen CL, Wang XK (2008) Appl Clay Sci 41:37
Khan SA, Riazurrehman, Khan MA (1994) Waste Manag 14:629
Xu D, Wang XK, Chen CL, Zhou X, Tan XL (2006) Radiochim Acta 94:429
Zhao DL, Feng SJ, Chen CL, Chen SH, Xu D, Wang XK (2008) Appl Clay Sci 41:17
Xu D, Shao DD, Chen CL, Ren AP, Wang XK (2006) Radiochim Acta 94:97
Wang XK, Chen CL, Zhou X, Tan XL, Hu WP (2005) Radiochim Acta 93:273
Hu J, Xu D, Chen L, Wang XK (2009) J Radioanal Nucl Chem 279:701
Tan XL, Wang XK, Geckeis H, Rabung T (2008) Environ Sci Technol 42:6532
Tan XL, Chen CL, Yu SM, Wang XK (2008) Appl Geochem 23:2767
Chen CL, Wang XK (2006) Ind Eng Chem Res 45:9144
Tan XL, Chang PP, Fan QH, Zhou X, Yu SM, Wu WS (2008) Colloid Surf A 328:8
Tan XL, Fan QH, Wang XK, Grambow B (2009) Environ Sci Technol 43:3115
Fan QH, Shao DD, Lu Y, Wu WS, Wang XK (2009) Chem Eng J 150:188
Kowal-Fouchard A, Drot R, Simoni E, Ehrhardt JJ (2004) Environ Sci Technol 38:1399
Shen GD, Hu J, Wang XK (2008) Appl Radiat Isot 66:1313
Chen CL, Wang XK, Nagatsu M (2009) Environ Sci Technol 43:2362
Tan XL, Wang XK, Fang M, Chen CL (2007) Colloid Surf A 296:109
Ibrahim HA, El-Kamash AM, Hanafy M, Abdel-Monem NM (2008) Chem Eng J 144:67
Chang PP, Wang XK, Yu SM, Wu WS (2007) Colloid Surf A 302:75
Acknowledgments
Financial supports from National Natural Science Foundation of China (20677058; 20501019) and Special Foundation for High-level Waste Disposal (2007-840) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, X., Wang, S., Yang, S. et al. Influence of contact time, pH, soil humic/fulvic acids, ionic strength and temperature on sorption of U(VI) onto MX-80 bentonite. J Radioanal Nucl Chem 283, 253–259 (2010). https://doi.org/10.1007/s10967-009-0323-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-009-0323-0