Abstract
Oxidized multiwalled carbon nanotubes (MWCNTs) were characterized by SEM and FTIR. The sorption of Th(IV) on MWCNTs was studied as a function of contact time, pH, ionic strength, Th(IV) concentration and temperature. The results indicate that the sorption of Th(IV) on MWCNTs is strongly dependent on pH and weakly dependent on ionic strength. The sorption thermodynamics of Th(IV) on MWCNTs was carried out at 293.15, 313.15 and 333.15 K, respectively, and the thermodynamic parameters (standard free energy changes (ΔG 0), standard enthalpy change (ΔH 0) and standard entropy change (ΔS 0)) were calculated from the temperature dependent sorption isotherms. The sorption of Th(IV) on MWCNTs is a spontaneous and endothermic process. The oxidized MWCNTs may be a promising candidate for the preconcentration and solidification of Th(IV), or its analogue actinides from large volumes of aqueous solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The environmental behavior of lanthanides and actinides has aroused great interest in terms of the radioactive waste disposal [1–4]. To assess the radionuclides behavior, radionuclide phenomena such as sorption, migration and diffusion in natural minerals and oxides are of paramount importance. The fate and transport of radionuclides in the environment is generally controlled by sorption reactions, complexation, colloid formation, etc. [5–10]. Thorium is only stable at its valence +IV in solution, and is usually used as a chemical analogue of other tetravalent actinides such as Np(IV), U(IV), and Pu(IV), which are difficult to study and to keep in the tetravalent form [11]. In recent decades, sorption of Th(IV) on different minerals and oxides have been studied extensively [12–18]. The results of the previous studies indicated that sorption of Th(IV) is strongly pH dependent and weakly ionic strength dependent [19–24]. However, the sorption capacity of Th(IV) on the minerals and oxides is still not high enough, which is crucial for the removal of Th(IV) from large volumes of aqueous solution.
Since the carbon nanotubes (CNTs) were discovered by Iijima [25] in 1991, the novel materials have come under intense multidisciplinary study because of their unique physicochemical properties [26–28]. CNTs include single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) depending on the number of layers comprising them. Because of their highly porous and hollow structure, large specific surface area, light mass density, and strong interaction between carbon and molecules, CNTs have been found as an efficient adsorbent for organic pollutants and heavy metal ions [29–34]. The earlier studies indicated that CNTs may be a promising adsorbent used in the environmental protection. But report concerning application of CNTs for radionuclide sorption was sparse. Wang et al. [35] firstly studied the sorption of 243Am(III) on MWCNTs and found that MWCNTs were a promising candidate for preconcentration and solidification of actinides from large radioactive solutions. The previous studies [36–38] demonstrated that the sorption of Sr(II), Am(III), and Eu(III) onto oxidized MWCNTs was affected by pH values and the sorption was mainly dominated by strong chemisorption and chemicomplexation. Belloni et al. [39] reviewed the application of MWCNTs in the removal of radionuclides from aqueous solutions, and conclude that MWCNTs can play a role in the fireld of nuclear waste management. However, the study of Th(IV) sorption on MWCNTs is still scarce [16], especially the effect of temperature on Th(IV) sorption.
Herein, we reported the application of MWCNTs in the removal of Th(IV) from aqueous solutions. The effect of pH, ionic strength and temperature on Th(IV) sorption was investigated. The thermodynamic parameters were calculated from the temperature dependent sorption isotherms, and the sorption mechanism was discussed.
Experimental
Materials
MWCNTs were prepared by using chemical vapor deposition (CVD) of acetylene in hydrogen flow at 760 °C using Ni–Fe nanoparticles as catalysts [35]. Oxidized MWCNTs were prepared by oxidization with 3 M HNO3 [35]. The surface area of oxidized MWCNTs is 197 m2/g by using the N2-BET method. The point of zero charge, pHpzc, i.e., the pH above which the total surface of the carbon is negatively charged, was measured at pH ~5 [35, 40].
1,000 mg L−1 Th(IV) stock solution was prepared as follow: ThO2 (purity >99.9%) was dissolved in 10 mL 3 M HNO3 and then transferred into a 500 mL vessel. The solubility of ThO2 was greatly improved by adding 2–3 drops HF (1:20) in the preparation of the thorium stock solution. The stock solution was diluted with Milli-Q water to obtain the desired concentrations. The concentration of Th(IV) was analyzed by spectrophotometry at wavelength 650 nm by using Th(IV) arsenazo(III) complex. The amount of Th(IV) sorption was calculated from the difference between the initial concentration and the equilibrium one.
Batch sorption experiments
Sorption kinetics was firstly carried out to achieve the equilibrium time. The experiments were performed at T = 293.15 ± 2 K in 0.01 M NaClO4 solution at stirring rate of 300 rpm. The stirring rate of 300 rpm was chosen to make MWCNTs homogenously to be dispersed in solution. Sorption isotherms were investigated by using batch technique in polyethylene centrifuge tubes under ambient conditions at 293.15 ± 2, 313.15 ± 2, and 333.15 ± 2 K, respectively. The blank experiments demonstrated that the sorption of Th(IV) on the test tube walls was negligible. The stock solutions of NaClO4 and MWCNTs were pre-equilibrated for 2 h before the addition of Th(IV) stock solution. The samples were gently shaken for 24 h (which was enough to achieve equilibrium). Duplicate batches were examined to achieve the sorption curves. The relative errors of the data were about ±5%.
Results and discussion
SEM and FTIR analysis
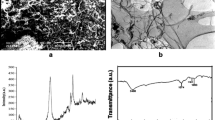
Figure 1 shows the scan electron microscope (SEM) image of oxidized MWCNTs. The oxidized MWCNTs have very smooth surfaces and cylindrical shapes with an external diameter of 10–30 nm. Due to inter-molecular force, the MWCNTs of different sizes and directions form an aggregated structure. The Fourier Transform infrared spectroscopy (FTIR) (Fig. 2) indicates that the acid treatment process introduces many functional groups onto the surfaces of MWCNTs: carbonyl groups (1,385 cm−1), carboxyl groups (1,580 cm−1), and hydroxyl groups (3,423 cm−1), which provide a large number of chemical adsorption sites [41]. Meanwhile, the hydrophilic properties of these functional groups improve the dispersivity of MWCNTs in aqueous solution [42].
Kinetic sorption study
Figure 3 shows the effect of contact time on Th(IV) sorption to MWCNTs. One can see that the sorption is rapid in the first 15 min of contact time, and 1 h is enough to achieve sorption equilibrium. The initial steep sorption curve suggests that the sorption occurs rapidly on the surface of MWCNTs. The sorption becomes slow subsequently because of the longer diffusion range of the Th(IV) ions (ionic radius ≈0.102 nm) into the inner channel (diameter ≈3.6 nm) of MWCNTs [35]. According to the above results, the shaking time was fixed for 24 h for the following batch sorption experiments.
Effect of pH and ionic strength
The sorption of Th(IV) on MWCNTs in 0.01 M NaClO4 solution as a function of pH is shown in Fig. 4. Sorption of Th(IV) is strongly dependent on pH values. Sorption of Th(IV) increases from ~10 to ~95% at pH 1–4, and then maintains its maximum level with increasing pH values. The results of Th(IV) sorption on hematite [43] indicated that half of Th(IV) was sorbed on hematite at pH = 2 and ~100% of Th(IV) was sorbed on hematite at pH = 4. Jakobsson [44] studied the sorption of Th(IV) on TiO2 and found that the sorption of Th(IV) started from pH 0.5 and increased quickly to ~100% at pH = 3, and concluded that reversible formation of an inner sphere complex with a strong pH dependence was the main sorption mechanism.
The sorption of Th(IV) on MWCNTs at pH 2.0 and 3.0 as a function of NaClO4 concentrations is shown in Fig. 5. One can see that the sorption of Th(IV) decreases a little with increasing NaClO4 concentration. With increasing Na+ concentrations in solution, the competitive sorption of Th(IV) with the cations adsorbed on MWCNTs increases and thereby the sorption of Th(IV) on MWCNTs decreases. Guo et al. [45] studied the sorption of Th(IV) on alumina and found that the sorption decreased drastically with increasing ionic strength at Th(IV) concentration of 7.5 × 10−5–2 × 10−4 mol/L; however, the sorption of Th(IV) increased with increasing ionic strength at Th(IV) concentration <7.5 × 10−5 mol/L. The pH and ionic strength dependent sorption of Th(IV) on MWCNTs suggests that the sorption of Th(IV) on MWCNTs can be attributed to surface complexation and cation exchange under our experimental conditions [46–48].
Sorption isotherms and thermodynamic parameters
The sorption isotherms of Th(IV) on MWCNTs at 293.15, 313.15 and 333.15 K are shown in Fig. 6a. One can see that the sorption isotherm is the highest at T = 333.15 K and is the lowest at T = 293.15 K. The results indicate that high temperature is advantageous for Th(IV) sorption on MWCNTs. This phenomenon is attributed to a steep simultaneous decrease of real sorption of solvent or a negative temperature coefficient of the solubility of solute [49]. Three different models, viz. Langmuir, Freundlich and D-R isotherm equations are conducted to simulate the sorption isotherms of Th(IV) on MWCNTs.
The Langmuir model assumes that sorption occurs in a monolayer with all sorption sites identical and energetically equivalent. Its linear form can be described as [50]:
where C eq is the equilibrium concentration of Th(IV) remained in solution (mol L−1); C s is the amount of Th(IV) adsorbed on MWCNTs after equilibrium (mol g−1); C s max is the maximum sorption capacity at complete monolayer coverage (mol g−1), and b (L mol−1) is a constant that relates to the heat of sorption.
The Freundlich expression is an exponential equation with the assumption that the sorption occurs on the heterogeneous sorbent surface. The equation is represented as [51]:
where k F (mol1−n Ln g−1) represents the sorption capacity when metal ion equilibrium concentration equals to 1, and n represents the degree of sorption dependent with equilibrium concentration.
The D-R model is more general than Langmuir model since it does not have the restriction of surface properties or constant sorption potential. The general expression is expressed as follow [51]:
where C s and C s max are defined above, β is the activity coefficient related to mean sorption energy (mol2 kJ−2), and ε is the Polanyi potential, which is expressed as:
where R is ideal gas constant (8.314 J mol−1 K−1), and T is the absolute temperature in Kelvin (K).
E (kJ mol−1) is defined as the free energy change, which requires to transfer 1 mol ions from aqueous solution to the solid surfaces. It can be calculated from the following equation:
The magnitude of E is used to evaluate the sorption mechanism. Sorption is dominated by chemical ion exchange if E is in the range of 8–16 kJ mol−1, whereas physical forces influences the sorption if E is lower than 8 kJ mol−1 [52].
The experimental data of Th(IV) sorption (Fig. 6a) are regressively simulated with Langmuir, Freundlich and D-R models and the results are shown in Fig. 6c–d, respectively. The relative values calculated from the three models are listed in Table 1. As can be seen from Fig. 6c–d, the three models simulate the sorption isotherms well, which is supported by the good correlation coefficients (all R 2 > 0.97 in Table 1). The fact that the sorption of Th(IV) on MWCNTs according with Langmuir model indicates that the binding energy on the whole surfaces of MWCNTs is uniform. This phenomenon also indicates that chemosorption is the principal sorption mechanism in sorption process. The E values obtained from Eq. 5 are 9.1 (T = 293.15 K), 10.0 (T = 313.15 K) and 10.4 kJ mol−1 (T = 333.15 K), which are in the sorption energy range of chemical ion-exchange reactions. The values of C s max obtained from the Langmuir model are the highest at T = 333.15 K and the lowest at T = 293.15 K, which indicates that the sorption is enhanced with increasing temperature. The value of n calculated from the Freundlich model is from unity, indicating that a nonlinear sorption of Th(IV) takes place on MWCNTs. It is necessary to note that the sorption capacities C s max derived from the D-R model are higher than those derived from the Langmuir model. This may be attributed to the different assumptions considered in the formulation of the isotherms. The parameters calculated from the analysis of the three isotherm models indicate that sorption of Th(IV) on attapulgite is a favorable and chemisorption process [53].
The distribution coefficients as a function of temperature at different initial concentrations at T = 293.15, 313.15 and 333.15 K are shown in Fig. 7. The thermodynamic parameters of ∆H 0, ∆S 0 and ∆G 0 of Th(IV) sorption on MWCNTs can be calculated from the temperature dependent sorption isotherms. The values of standard enthalpy change (∆H 0) and standard entropy change (∆S 0) can be calculated from the slope and y-intercept of the plot of lnK d versus 1/T (Fig. 7) using the following equations [41]:
Gibbs free energy changes (∆G 0) of specific adsorption are calculated from:
Relevant parameters calculated from Eqs. 7 and 8 are given in Table 2. The values of thermodynamic parameters provide an insight into the mechanism concerning the interaction of Th(IV) on MWCNTs. The values of ∆H 0 are positive, which suggests that the sorption process is endothermic. One possible interpretation of the endothermic process is that Th(IV) ions are well solvated in water [40]. In order for Th(IV) ions to adsorb, they are denuded of their hydration sheath to some extent, and this dehydration process needs energy. It is assumed that this energy of dehydration exceed the exothermicity of Th(IV) ions attaching to MWCNTs. The removal of water molecules from Th(IV) ions is essentially an endothermic process, and the endothermicity of the desolvation process exceed the enthalpy of sorption to a considerable extent [54]. The Gibbs free energy change (∆G 0) is negative as expected for a spontaneous process. The decrease of ∆G 0 with increasing temperature indicates more efficient sorption at higher temperature. Th(IV) ions are readily desolvated at higher temperature, and thereby their sorption to MWCNTs becomes more favorable. The positive values of entropy change (∆S 0) reflect the affinity of MWCNTs toward Th(IV) ions in aqueous solutions and might suggest some structure changes.
Conclusion
The sorption of Th(IV) on oxidized MWCNTs was studied under ambient conditions. The effect of contact time, pH, ionic strength and temperature on Th(IV) sorption to MWCNTs was investigated. The pH and ionic strength dependent sorption suggests that the sorption of Th(IV) on MWCNTs is mainly dominated by surface complexation and ion exchange. The oxygen-containing functional groups of MWCNTs contribute to Th(IV) sorption on MWCNTs. The fast sorption of Th(IV) on MWCNTs suggests that MWCNTs can be used in the removal of Th(IV) ions from large volumes of aqueous solutions. The high removal of Th(IV) from solution to MWCNTs suggests that MWCNTs are suitable materials in the preconcentration of Th(IV) ions in real work in nuclear waste management.
References
Tan XL, Fan QH, Wang XK, Grambow B (2009) Environ Sci Technol 43:3115
Chen CL, Wang XK, Nagatsu M (2009) Environ Sci Technol 43:2362
Fan QH, Tan XL, Li JX, Wang XK, Wu WS, Montavon G (2009) Environ Sci Technol 43:5776
Tan XL, Wang XK, Geckeis H, Rabung Th (2008) Environ Sci Technol 42:6532
Chen CL, Xu D, Tan XL, Wang XK (2007) J Radioanal Nucl Chem 273:227
Seco F, Hennig C, Pablo J, Rovira M, Rojo I, Marti V, Gimenez J, Duro L, Grive M, Bruno J (2009) Environ Sci Technol 43:2825
Ren XM, Wang SW, Yang ST, Li JX (2010) J Radioanal Nucl Chem 283:253
Hu J, Xu D, Chen L, Wang XK (2009) J Radioanal Nucl Chem 279:701
Tan XL, Fang M, Wang XK (2010) Molecules 15:8431
Ayata S, Aydinci S, Merdivan M, Binzet G, Kulcu N (2010) J Radioanal Nucl Chem 285:525
Chen CL, Wang XK (2007) Appl Geochem 22:436
Sabale SR, Jadhav DV, Mohite BS (2010) J Radioanal Nucl Chem 284:273
Bursali EA, Merdivan M, Yurdakoc M (2010) J Radioanal Nucl Chem 283:471
Qian LJ, Zhao JN, Hu PZ, Geng YX, Wu WS (2010) J Radioanal Nucl Chem 283:653
Zhang HX, Yang C, Tao ZY (2009) J Radioanal Nucl Chem 279:317
Chen CL, Li XL, Wang XK (2007) Radiochim Acta 95:261
Tan XL, Wang XK, Fang M, Chen CL (2007) Colloid Surf A 296:109
Yu SM, Chen CL, Chang PP, Wang TT, Lu SS, Wang XK (2008) Appl Clay Sci 38:219
Sheng GD, Hu J, Wang XK (2008) Appl Radiat Isot 66:1313
Zhao DL, Feng SJ, Chen CL, Chen SH, Xu D, Wang XK (2008) Appl Clay Sci 41:17
Zhang HX, Yuan JQ, Tao ZY (2007) J Radioanal Nucl Chem 273:495
Humelnicu D, Drochioiu G, Sturza MI, Cecal A, Popa K (2006) J Radioanal Nucl Chem 270:637
Salinas-Pedroza MG, Olguin MT (2004) J Radioanal Nucl Chem 260:115
Guo ZJ, Niu LJ, Tao ZY (2005) J Radioanal Nucl Chem 266:333
Iijima S (1991) Nature (London) 354:56
Chen CL, Hu J, Xu D, Tan XL, Meng YD, Wang XK (2008) J Colloid Interf Sci 323:33
Tan XL, Fang M, Chen CL, Yu SM, Wang XK (2008) Carbon 46:1741
Ren XM, Chen CL, Nagatsu M, Wang XK (2011) Chem Eng J. doi:10.1016/j.cej.2010.08.045
Tan XL, Xu D, Chen CL, Wang XK, Hu WP (2008) Radiochim Acta 96:23
Xu D, Tan XL, Chen CL, Wang XK (2008) J Hazard Mater 154:407
Shao DD, Jiang ZQ, Wang XK, Li JX, Meng YD (2009) J Phys Chem B 113:860
Hu J, Chen CL, Zhu XX, Wang XK (2009) J Hazard Mater 162:1542
Yang ST, Li JX, Shao DD, Hu J, Wang XK (2009) J Hazard Mater 166:109
Sheng GD, Shao DD, Ren XM, Wang XQ, Li JX, Chen YX, Wang XK (2010) J Hazard Mater 178:505
Wang XK, Chen CL, Hu WP, Ding AP, Xu D, Zhou X (2005) Environ Sci Technol 39:2856
Chen CL, Hu J, Shao DD, Li JX, Wang XK (2009) J Hazard Mater 164:923
Shao DD, Hu J, Wang XK (2010) Plasma Process Polymer 7:977
Fan QH, Shao DD, Hu J, Chen CL, Wu WS, Wang XK (2009) Radiochim Acta 97:141
Belloni F, Kutahyali C, Rondinella VV (2009) Environ Sci Technol 43:1250
Li JX, Chen SY, Sheng GD, Hu J, Tan XL, Wang XK (2011) Chem Eng J 166:551
Sheng GD, Li JX, Shao DD, Hu J, Chen CL, Chen YX, Wang XK (2010) J Hazard Mater 178:333
Chen CL, Liang B, Ogino A, Wang XK, Nagatsu M (2009) J Phys Chem C 113:7659
Reiller P, Casanova F, Moulin V (2005) Environ Sci Technol 39:1641
Jakobsson AM (1999) J Colloid Interf Sci 220:367
Guo ZJ, Yu XM, Guo FH, Tao ZY (2005) J Colloid Interf Sci 288:14
Shao DD, Hu J, Sheng GD, Ren XM, Chen CL, Wang XK (2010) J Phys Chem C 114:21524
Hu J, Shao DD, Chen CL, Sheng GD, Ren XM, Wang XK (2011) J Hazard Mater 185:463
Zeng YH, Liao XP, He Q, Shi B (2009) J Radioanal Nucl Chem 280:91
Shao DD, Ren XM, Hu J, Chen YX, Wang XK (2010) Colloid Surf A Physicochem Eng Aspects 360:74
Hu J, Shao DD, Chen CL, Sheng GD, Li JX, Wang XK, Nagatsu M (2010) J Phys Chem B 114:6779
Hu BW, Cheng W, Zhang H, Sheng GD (2010) J Radioanal Nucl Chem 285:389
Zhao GX, Zhang HX, Fan QH, Ren XM, Li JX, Chen YX, Wang XK (2010) J Hazard Mater 173:661
Lu SS, Xu JZ, Zhang CC, Niu ZW (2011) J Radioanal Nucl Chem. doi:10.1007/s10967-010-0849-1
Genc-Fuhrman H, Tjell JC, Mcconchie D (2004) Environ Sci Technol 38:2424
Acknowledgments
Financial supports from Anhui Province Department of Education Fund (2006KJ123, 20101941) and Anhui Province Department of Health Medical Science Fund (2010C081) are acknowledged. We also thank Prof. X. Wang (IPP, China) for providing us the samples of MWCNTs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, M., Tao, X. & Song, X. Effect of pH, ionic strength and temperature on sorption characteristics of Th(IV) on oxidized multiwalled carbon nanotubes. J Radioanal Nucl Chem 288, 859–865 (2011). https://doi.org/10.1007/s10967-011-1007-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1007-0