Abstract
Previous studies including the development of methods for the determination of carbon, nitrogen, and phosphorus in cattail using cold neutron prompt gamma activation (CNPGAA) and thermal neutron prompt gamma activation analysis (TNPGAA); evaluation of the precision and accuracy of these methods through the analysis of Standard Reference Materials (SRMs); and comparison of the sensitivity of CNPGAA to TNPGAA have been done in the CNPGAA and TNPGAA facilities at the National Institute of Standards and Technology (NIST). This paper integrates the findings from all of these prior studies and presents recommendations for the application of CNPGAA and TNPGAA in environmental studies of plants based on synergistic considerations of the effects of neutron energy, matrix factors such as chlorine content, Compton scattering, hydrogen content, sample thickness, and spectral interferences from Cl on the determination of C, N, and P. This paper also provides a new approach that simulates a sensitivity curve for an element of interest (S), which is a function of hydrogen content (X) and sample thickness (Y) as follows: S = aX + bY + c (where a, b, and c are constants). This approach has provided more accurate results from the analysis of SRMs than traditional methods and an opportunity to use models to optimize experimental conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cattails (Typha domingensis) have received considerable attention by scientists and agencies in Florida and around the U.S. over the past century. In the Florida Everglades, these aquatic invasive plants expand dramatically into marshes and wetlands and replace native plant sawgrass as a result of nutrient input from agricultural runoff [1–3]. Early signs of this expansion are apparent in the ecosystem of Apalachicola National Estuarine Research Reserve (ANERR) located in northwest Florida (USA). To investigate the growth of cattail (Typha domingensis) in the ANERR ecosystem, the development of methods for the determination of carbon, nitrogen, and phosphorus in cattail (Typha domingensis) using cold neutron prompt gamma activation (CNPGAA) and thermal neutron prompt gamma activation analysis (TNPGAA); evaluation of the precision and accuracy through the analysis of Standard Reference Materials (SRMs); and comparison of the sensitivity of CNPGAA to TNPGAA have been done in the CNPGAA and TNPGAA facilities at the National Institute of Standards and Technology (NIST) [4–7].
Previous studies [4–6] show that the differences in concentrations of C and N in SRMs obtained by using CNPGAA, TNPGAA, and an elemental analyzer (EA) when compared to the certified values were less than 0.2%, 0.3%, and 3%, respectively while the differences in concentrations of P in SRMs obtained by using CNPGAA and TNPGAA when compared to the certified values were less than 13% and 0.1%, respectively. The differences in concentrations of C and N comparing CNPGAA and TNPGAA to EA were less than 4.5% and 6.1%, and 1.6% and 4.2%, respectively. However, the results of cattail for P obtained by CNPGAA overestimated the results from a UV–VIS spectrophotometer by a factor of 7.9 ± 0.7 (n = 12) (L. Zhao, Unpublished data) although the difference of P concentrations obtained by using CNPGAA when compared to the certified value in SRM1570a-Spinach Leaves, SRM1573a-Tomato Leaves, SRM1515-Apple Leaves, and SRM1575a-Pine Needles, were less than 2% [6]. This is because the above SRMs are terrestrial plants with Cl concentrations less than 1% while the cattails are aquatic plants which grow in brackish water at the study site and have an average Cl concentration of 5.8% (range 4.2–8.1%) (L. Zhao, Unpublished data). Therefore, the spectral interference from Cl (637.7 keV) [8] in the cattails has a major impact on the detection of P (636.7 keV) [8] although it did not influence the SRMs [4, 6] because of their lower Cl concentrations. There are also other effects including neutron energy, Compton scattering, hydrogen content, and sample thickness on the sensitivities of C, N and P in CNPGAA and TNPGAA. This paper integrates the findings from all of these prior studies and presents recommendations for the application of CNPGAA and TNPGAA in environmental studies of plants based on synergistic considerations of these effects on the determination of C, N, and P. This paper also provides a new approach that simulates a sensitivity curve for an element of interest (S), which is a function of hydrogen content (X) and sample thickness (Y) as follows: S = aX + bY + c (where a, b, and c are constants). This approach has provided more accurate results from analysis of SRMs than traditional methods and an opportunity to use models to optimize experimental conditions [4–7].
Experimental
Sample collection and preparation
Cattails were collected from 24 locations within the 8 sampling stations located in ANERR during the wet season, growing season, and dry season in 2002 and 2003, and prepared as a fine powder (<200 mesh) for analysis through a series of the procedures as described in our previous studies [4–6].
CNPGAA and TNPGAA procedures
Prompt gamma-ray activation analysis using thermal neutrons (TNPGAA) has been used for the determination of light elements in biological materials [9–12]. Although PGAA instruments using guided beams of cold (low energy) neutrons offer the advantage of better detection limits and sensitivities for most elements than thermal neutron instruments [13], CNPGAA has not been used widely for the analysis of biological materials because of the effects of neutron scattering by hydrogen in the sample.
Typically, results from CNPGAA [14, 15] and TNPGAA [16] can be made more accurate by matching the sample thickness and hydrogen content between samples and standards. This is suitable for analysis of a homogeneous and single sample, but it is not possible in our studies [4–6]. First, it is not possible to prepare a universal standard to match the same sample thickness in practice while maintaining the same H content in samples such as SRMs and cattails because of their different densities. Second, it is also not possible to prepare a universal standard to match the same H content in practice while maintaining the same sample thickness in cattail, which had varying H content (5.4–5.9%). Therefore, a new method in which sensitivity curves of C, N, and P were simulated using a group of standards with different sample thickness and H content was proposed in the previous studies [4–6]. The results obtained from the sensitivity curves were better than the results using the average sensitivity [4, 5]. Based on our pilot studies it was assumed that the sensitivities of C, N, and P varied proportionally with H content and sample thickness around the range of H content in filed samples as follows:
where S is sensitivity for element of interest; X is H content; Y is sample thickness, and a, b, and c are constants. Sensitivity for an element of interest contains three parts: (1) aX is the amount of sensitivity that is enhanced or decreased as a function of H content while a represents the changing rate of sensitivity with varying H content; (2) bY is the amount that sensitivity is either enhanced or decreased as a function of sample thickness while b represents the changing rate of sensitivity with varying sample thickness, and (3) c is the sensitivity obtained in the absence of neutron scattering by hydrogen and neutron self-shielding. The constants a, b, and c can be obtained by a least squares fit from a group of standards with varying H content and sample thickness.
Standards for C, H, N, and P were prepared from mixtures of monomethyl phosphate di-cyclohexylammoniun salt (CH5O4P·2C6H13N), cellulose (C6H10O5), silicon dioxide (SiO2), graphite (C), mannitol (C6H8(OH)6), and urea (CH4N2O) as pellets as described in the previous studies [4–6]. Cattail and SRMs samples were also prepared as pellets.
Samples were irradiated for 7 h (thermal equivalent neutron flux of 9 × 108 neutrons cm−2 s−1), and 16 K spectra up to 11 MeV with Compton suppression on or off were collected using a high purity germanium (HPGe) detector, a 16 K × 32-bit analog-to-digital converter (ADC) and an acquisition interface module (AIM) operated under a DEC VAX station. Neutron fluence was monitored for normalizing count rates of C, N, P, and H during sample collection.
Spectra were transformed as a text file using the Fullist code developed at NIST (R.M. Lindstrom, Unpublished code). A Microsoft Excel spreadsheet was used for fitting calibration curves and subsequent calculations of peak area integration [17], concentration, uncertainty, neutron fluence rates, pileup and background corrections.
Results and discussion
To compare the effects of energy, matrix factors such as chlorine (Cl), Compton scattering, and spectral interferences from Cl on the determination of C, N, and P in CNPGAA, spectra of a blank, standard, SRM, and cattail are shown in Fig. 1.
As shown in Fig. 1A, there are much higher sensitivities for Cl in CNPGAA and TNPGAA than other elements (C, N, and P) so that all major strongest peaks in the spectra of the cattail and SRM were from Cl except for H. The seven strongest peaks of Cl were distributed in the energy range of 0–6,000 keV. However, five of these peaks were distributed in the energy range of 0–2,500 keV (Fig. 1B). Comparing the spectra of cattail and the SRM in Fig. 1B, the cattail had higher background than the SRM although they had a similar H content. Therefore, the peaks of Cl are a major contributor to the higher background in cattail by Compton scattering. This suggests that it is the best way to choose peaks for N, C, and P beyond 2,500 keV for analysis.
Comparing the cattail spectra with Compton suppression on (Cattail (Compton on) in Fig. 1B) to that with Compton suppression off (Cattail (Compton off) in Fig. 1B), the cattail spectra with Compton suppression off had much higher background than the cattail spectra with Compton suppression on, and the Compton edges (CE-Cl1, CE-Cl2, CE-Cl3, CE-Cl5, and CE-H in Fig. 1B) of full energy peaks (Cl1, Cl2, Cl3, Cl5, and H) with Compton suppression on were much smoother than that with Compton suppression off. Therefore, this suggests that it is the best way to choose Compton suppression on in order to reduce the background caused by Compton scattering. However, as shown in Fig. 1D, for nitrogen the cattail spectra with Compton suppression on had only two peaks (full energy (Full) and single escape (SE) peak) because of the double escape peak disappearing as Compton suppression was on. Nitrogen in the cattail spectra with Compton suppression off had three peaks (full energy (Full), single (SE), and double escape (DE) peaks). Therefore, the nitrogen sensitivity with Compton suppression off was much higher than that with Compton suppression on because of calculating the nitrogen sensitivity with Compton suppression off including three peaks (full energy (Full), single (SE), and double escape (DE) peaks) while calculating the nitrogen sensitivity with Compton suppression on only including two peaks (full energy (Full) and single escape (SE) peak). Therefore, it is the best way to choose Compton suppression off in determination of N in cattail as a result of increasing nitrogen sensitivity.
Based on considerations of spectral interferences from C, N, and other elements in the matrix of samples on P detection, the P peak was chosen at E = 636.7 keV and evaluated through SRMs. Previous studies shows that the difference of P concentrations obtained by using CNPGAA when compared to the certified value in SRM1570a-Spinach Leaves, SRM1573a-Tomato Leaves, SRM1515-Apple Leaves, and SRM1575a-Pine Needles, were less than 2% [6]. However, the results of cattail for P obtained by CNPGAA overestimated the results from a UV–VIS spectrophotometer (L. Zhao, Unpublished data). As shown in Fig. 1C the P peak in cattail was higher than that in the SRM although the SRM had higher concentrations of P than the cattail. This is caused by the interference of Cl (E = 637.6 keV) [8] because the cattails have higher Cl concentrations (4.2–8.1%) while the above SRMs have lower Cl concentrations (<1%) (L. Zhao, Unpublished data). Therefore, as samples contain higher Cl concentrations it is necessary to consider the interference of Cl (E = 637.6 keV) [8] on P detection. It can be resolved well as previous studies [7].
Therefore, it is the best way to choose E = 636.7 keV, 1261.7 keV, and 10829.2 keV for analysis of P, C, and N, respectively, with Compton suppression off in the determination of C, N, and P using CNPGAA based on synergistic consideration of the effects of energy, matrix factors, Compton scattering, and spectral interferences from Cl.
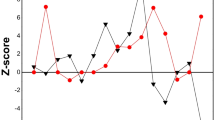
As shown in Fig. 2, the sensitivities of P, C, and N decreased in CNPGAA while the sensitivities of P and N increased and the sensitivity of C decreased in TNPGAA as the H content increased. There are two major effects of neutron scattering by hydrogen in CNPGAA: redirection of the path of the neutron and transfer of energy to and from the neutron to the scattering nucleus (almost entirely hydrogen). The first effect can either increase or decrease the average path length of the neutron within the sample [18, 19]. An increase in the average path length will increase the probability of interaction while a decrease in the average path length of the neutron within the sample will decrease the probability of interaction [18, 19]. This effect (either increase or decrease in signal) is independent of neutron energy (temperature). At the NIST facility, the sample is oriented at about a 45° angle to the beam. The net result of neutron scattering for this geometry (redirection of neutron direction) will be a net increase in signal in CNPGAA. The second effect (energy transfer) on average results in decreased cross sections by the increased energy of the neutron. Since the sample is at about 300 K and the beam is about 40 K there will be a net increase of neutron energy resulting from scattering effects (unless the sample is cooled to temperatures similar to the beam). For CNPGAA, energy transfer is a much bigger negative effect (decrease in signal) than the positive effect of redirection of the path of the neutrons. That is the reason that the overall effect of neutron scattering by hydrogen in samples decreases the sensitivities of C, N, and P in CNPGAA at NIST. However, there is only one major effect of neutron scattering by H in TNPGAA: redirection of the path of the neutron. Although the TNPGAA facility has a similar geometry of the CNPGAA facility, the net result of neutron scattering by hydrogen (redirection of neutron direction) is a net increase in signal for P and N while it will be a net decrease in signal for C in TNPGAA. This is the reason that the effect of neutron scattering by hydrogen in samples increases the sensitivities of N and P and decreases the sensitivity of C in TNPGAA at NIST. As shown in Fig. 2, the sensitivities of C, N, and P decreased as the sample thickness increased in CNPGAA and TNPGAA. This is caused by neutron self-shielding.
Although Fig. 2 shows the effects of H content and sample thickness on sensitivities of C, N, and P qualitatively, the sensitivity curve can show the relationships among sensitivity, H content, and sample thickness quantitatively and in detail. As shown in previous work [7], the sensitivity curves of P, C, and N with Compton suppress on or off in CNPGAA and TNPGAA (S P, S C, and S N) are presented as follows:
By comparing the ratio of constant a to b in Eq. 1 from Eqs. 2-1, 2-3, 2-5, and 2-6 for CNPGAA (a CNPGAA/b CNPGAA = 19.7, 1.9, 1.7, and 1.1) [7], as H content and sample thickness increased by one unit, the effect of sample thickness on the sensitivity of P was much greater than the effect of H content (Compton suppress on or off); the effect of H content on the sensitivity of C was slightly greater than the effect of sample thickness when Compton suppression was on or off; the effect of sample thickness on the sensitivity of N was much greater than the effect of H content (Compton suppression off); and the effect of H content on the sensitivity of N was slightly greater than the effect of sample thickness (Compton suppression on). Similar to TNPGAA (a TNPGAA/b TNPGAA = 28.2, 20.2, and 1.1 from Eqs. 2-2, 2-4, and 2-7, respectively) [7], as H content and sample thickness increased by one unit (Compton suppression on), the effect of sample thickness was much greater than the effect of H content on the sensitivity of P; the effect of sample thickness was much greater than the effect of H content on the sensitivity of C; and the effect of H content was slightly greater than the effect of sample thickness on the sensitivity of N. Therefore, the overall effect of the sample thickness is greater than the effect of H content on sensitivities of C, N, and P in CNPGAA and TNPGAA.
A comparison of the synergistic effects of H content and sample thickness on sensitivities of C, N, and P between CNPGAA and TNPGAA from Eq. 2 is shown in Fig. 3 [7]. As shown in Fig. 3, overall sensitivities of P, C, and N in CNPGAA were greater than that in TNPGAA by factors of 2.2(65 mg H, 1.5 mm) to 47.7(15 mg H, 4.5 mm); 2.8(65 mg H, 1.5 mm) to 5.5(15 mg H, 4.5 mm); and 5.6(65 mg H, 1.5 mm) to 22.9(15 mg H, 4.5 mm), respectively. The overall sensitivity of N with Compton suppression off was greater than that with Fig. 3 Compton suppression on by a factor of 1.5(65 mg H, 4.5 mm) to 2.5(15 mg H, 1.5 mm).
Figure 3 also provides a useful visual tool to demonstrate the effects of H content and sample thickness on sensitivities. Therefore, the sensitivity curves, simulated from experimental data, can be used to optimize experimental conditions.
Conclusion
Based on synergistic considerations of the effects of energy, matrix factors such as chlorine (Cl) content, Compton scattering, hydrogen content in plants, sample thickness, and spectral interferences from Cl on the determination of C, N, and P in cattail, CNPGAA with Compton suppression off is the best method for determination of P, C, and N in cattail in the CNPGAA facilities at NIST. This also demonstrates an example of the analysis environmentally relevant plants using CNPGAA. A sensitivity curve also provides an opportunity to use models to optimize experimental conditions.
References
Miao SL, Sklar FH (1998) Wetlands Ecol Manage 5:245
Chiang C, Craft CB, Rogers DW, Richardson CJ (2000) Aquat Bot 68:61
Miao SL, Debusk WF (1999) Phosphorus biogeochemistry in subtropical ecosystems. Lewis Publishers, Boca Raton, FL, p 275
Zhao L, Robinson L, Paul RL, Greenberg RR, Miao SL (2005) J Radioanal Nucl Chem 263:805
Zhao L, Robinson L, Mackey EA, Paul RL, Greenberg RR (2008) J Radioanal Nucl Chem 277:275
Zhao L, Robinson L, Paul RL, Greenberg RR, Miao SL (2007) J Radioanal Nucl Chem 271:777
Zhao L, Robinson L (2009) J Radioanal Nucl Chem. doi:10.1007/s10967-009-0309-y
NNDC, National Nuclear Data Center (2009) http://www.nndc.bnl.gov/capgam/indexbye.html
Mackey EA (1994) Biol Trace Elem Res 43–45:103
Anderson DL, Cunningham WC, Lindstrom TR (1994) J Food Comp Anal 7:59
Anderson DL, Cunningham WC, Alvarez GH (1993) J Radioanal Nucl Chem 167:139
Anderson DL, Cunningham WC (1994) Trans Am Nucl Soc 71:21
Paul RL, Lindstrom RM (2000) J Radioanal Nucl Chem 243:181
Paul RL (1995) J Radioanal Nucl Chem 191:245
Mackey EA, Gordon GE, Lindstrom RM, Anderson DL (1992) Anal Chem 64:2366
Paul RL (1997) Analyst 122:35R
Lindstrom RM (1994) Biol Trace Elem Res 43–45:597
Mackey EA, Copley JRD (1993) J Radioanal Nucl Chem 167:127
Johnson E, Robinson L (1989) Rev Sci Instr 60:3447
Acknowledgements
Financial support for this project was provided by the U.S. Department of Education Title III program, the National Oceanic and Atmospheric Administration Education Partnership Program’s Environmental Cooperative Science Center housed at Florida A&M University, and the U.S. Department of Energy through a program administered by Oak Ridge Associated Universities. Many thanks to R. L. Paul, E. A. Mackey, R. R. Greenberg, and R. M. Lindstrom at NIST; S. L. Miao at Everglades Department, South Florida Water Management District, E. Johnson, R. Gragg, and K. Milla at Florida A&M University; and L. Edmiston, C. Bedee, L. Levi, and K. Miller at the Apalachicola National Estuarine Research Reserve.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robinson, L., Zhao, L. Effects of sample and spectrum characteristics on cold and thermal neutron prompt gamma activation analysis in environmental studies of plants. J Radioanal Nucl Chem 282, 199–204 (2009). https://doi.org/10.1007/s10967-009-0310-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-009-0310-5