Abstract
A method for continuous determination of the light rare earth elements (LREEs) and thorium in Baotou Iron Ore was established. The light rare earths and thorium were adsorbed on a micro-column packed with HD-8 cation exchange resins. The light rare earth elements were eluted with 4 mol L−1 HCl–2 mol L−1 NH4Cl solution and determined with tribromo-arsenzao by a 721-E spectrophotometry at 630 nm; thorium was eluted with 5% potassium oxalate solution and determined with Arsenazo III by a 721-E spectrophotometry at 660 nm. The measured values by the proposed method were in close agreement with the certified values (Baotou main ore standard sample, Baotou ore R-715 standard sample and GSD-2 standard sample). The RSD of the light rare earths and thorium in Baotou Iron Ore were of <1.70% and <1.99%, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The content of the rare earth elements in Baotou Iron Ore in Mongolia is especially high, in which the resources of the rare earth elements rank first in the world and thorium second. The rare earth elements and thorium have been widely applied to industry, agriculture and nuclear power plant. Thus, one of the researchers’ interest is how to separate and determinate the rare earth elements and thorium from ore.
Numerous separation and preconcentration procedures, such as liquid-liquid extraction [1, 2] and solid-phase extraction (SPE) [3–6], ion-exchange [7, 8], have been developed for metal ions. SPE, especially on-line separation and preconcentration, has drawn extensive attention, because of representing a link between solvent extraction and ion-exchange, and operating easily, quickly, only requiring a small amount of eluent.
Various papers of separation of the REEs and thorium were reported, such as organophosphorus reagent [9], amide compound [10, 11] for thorium, and sillica gel [12], ion-exchange resin [13] for the rare earth elements. Likewise, there were many papers on the determination of the REEs and thorium, for instance, spectrophotometry [14], ICP-AES [15], ICP-MS [16–18], instrumental neutron activation analysis (INAA) [16, 19]. However, the separation and determination of the REEs and thorium [6, 20] were achieved by fusing ore separately. There were fewer articles on simultaneous separation and preconcentration of the REEs and thorium [21].
The aims of this work are to establish a method of continuous separation and determination of the REEs and thorium, to study the elution conditions, and to apply this technique to determine the REEs and thorium in Baotou Iron Ore.
Experimental
Reagents and chemicals
A 20 μg mL−1 LREEs solution was prepared by addition of 1,000 μg La, 2000 μg Ce, 200 μg Pr, 800 μg Nd and 16 mL of concentrated hydrochloric acid into a 200-mL volumetric flask, diluted to the mark with deionized water. A 1 mg mL−1 thorium stock solution was prepared by dissolving 1.1895 g of spectral purity thorium nitrate in 10 mL of concentrated hydrochloric acid. The mixture was diluted to 500 mL with hydrochloric acid and deionized water. A 10 μg mL−1 thorium standard solution was prepared by diluting the requisite amount of standard thorium stock solution to volumetric flask. Unless stated otherwise, all the chemicals used were of analytical reagent grade; deionized water (18 MΩ) was used throughout this study. A sufficient amount HD-8 resins (140–160 mesh, provided by HuaChang polymer Ltd. of East China University of Science and Technology, Shanghai, China) were dipped with deionized water for 24 h.
Apparatus
A 721-E spectrophotometer (Shanghai, China) was used for the determination of the LREEs and thorium using tribromo-arsenzao at 630 nm and Arsenazo III at 660 nm, respectively. A peristaltic pump (HL-1, Shanghai Huxi Instrumentation Factory, Shanghai, China) was used to control the flow rate. Some prepared hollow glass pipes (2.8 mm in diameter and 70 mm in length) filled with the prepared HD-8 resins, were used for the experiments. The ends of the column were plugged with glass wool to retain the HD-8 resins in the column.
General operation procedures
The prepared HD-8 resin column was first washed with deionized water and then conditioned with 1 mol L−1 HCl. The solution containing the LREEs and thorium was passed through the column at the optimum flow rate, controlled by a peristaltic pump. A small amount of 1 mol L−1 HCl was used to wash the column. A volume of 4 mL of 4 mol L−1 HCl–2 mol L−1 NH4Cl (1 mL a time) was used to desorb the LREEs into a 25-mL volumetric flask and the volume was diluted to the mark before determination. The column was rinsed with 2 mL of 20% NH4Cl (1 mL a time) and 3 mL of deionized water (1 mL a time). A volume of 3 mL of ammonium oxalate solution (1 mL a time) was used to desorb thorium into a 10-mL test tube which was then diluted to the mark before determination.
Determination of the LREEs and thorium
The LREEs and thorium were detected using tribromo-arsenzao as a chromogenic reagent at 630 nm on a 721-E spectrophotometer and Arsenazo III as a chromogenic reagent at 660 nm on a 721-E spectrophotometer, respectively.
Results and discussion
Effect of hydrochloric acid concentration on adsorption of the LREEs and thorium
Hydrochloric acid solution was used to condition the column before use. In this section, the effect of the concentration of the hydrochloric acid solution on adsorption was investigated by changing the concentration of hydrochloric acid solution from 1 to 4 mol L−1.
The adsorption of the LREEs and thorium were nearly 100% when the concentrations of hydrochloric acid range from 1 to 4 mol L−1. In order to insure the adsorption of the LREEs and esay-operation, 1 mol L−1 HCl solution was used in the experiment.
Effect of the transformed solution on thorium
When the column was passed through thorium solution and washed with 1 mol L−1 HCl, the column must be transformed. The types as well as the volumes of the transformed solutions were used to study the effect of transformed solution on thorium.
Both 20% NH4Cl and 20% KCl can be used as transformed solution (Fig. 1), 1–2 mL of solution has little effect on the adsorption rate of thorium. Two milliliters of 20% NH4Cl was used as the transformed solution.
Elution of thorium
In this section, the effect of the types as well as volumes of the eluent on the absorbance was studied. A series of 4% H2C2O4, 4% (NH4)2C2O4 and 5% K2C2O4 solution of different volumes were tested. A portion of 2 mL of 10 μg mL−1 standard thorium solution was passed through the column.
It is clear that both 3 mL of 5% K2C2O4 and 3 mL of 4% (NH4)2C2O4 can entiretily elute thorium without drag tail (curve 1, curve 2 in Fig. 2). But the solubility of (NH4)2C2O4 solution is low and it crystallizes easily when the temperature is below 10 °C. K2C2O4 solution can easily combine with thorium. Thus, 3 mL of 5% K2C2O4 was selected as the eluent for thorium.
Effect of the eluent on the LREEs
Two milliliters of 20 μg mL−1 LREEs solution was passed through the column. Different eluents (4 mol L−1 HCl–2 mol L−1 NH4Cl solution and 6 mol L−1 HCl solution) were used for eluting the LREEs.
Four mol L−1 HCl–2 mol L−1 NH4Cl can elute the low LREEs completely and the volume of the eluent was fewer than that of 6 mol L−1 HCl (Fig. 3). So the solution of 4 mol L−1 HCl–2 mol L−1 NH4Cl was used as the eluent for the LREEs.
The content of the LREEs in Baotou Iron Ore is much higher than that of thorium. The eluent of the high content LREEs must be investigated. Four mol L−1 HCl–2 mol L−1 NH4Cl was used to elute the high content LREEs (1 mg).
It was evident that the absorbance of the LREEs decreased with the increase of the volumes of 4 mol L−1 HCl–2 mol L−1 NH4Cl (Fig. 4). One milligram LREEs can be eluted completely with 8 mL of 4 mol L−1 HCl–2 mol L−1 NH4Cl. Thus, 8–10 mL of 4 mol L−1 HCl–2 mol L−1 NH4Cl was used as the eluent for the high content LREEs (>1 mg) ensuring the separation of the LREEs and thorium.
Effect of the mass ratio of the LREEs and thorium on the recovery of the LREEs and thorium
The recovery of the LREEs and thorium was affected with different mass ratios. The appropriate mass ratio must be studied because of the difference of the content of the LREEs and thorium in different areas. The series of 2:1, 10:1, 20:1, 50:1 and 100:1 of different mass ratios of the LREEs and thorium were tested.
The recovery of the LREEs and thorium were all above 97% when the mass ratios were changed from 2:1 to 100:1. Eight to ten milliliters of the eluent was sufficient to elute the LREEs and thorium of high mass ratio (>100:1), and the breakthrough of thorium did not happen, thorium adsorbing on the column firmly.
Effect of the main interfered ions
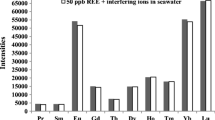
The experiment was achieved by addition of different contents of the interfered ions as well as 20 μg LREEs and 10 μg thorium. When the deviation was ±5%, the allowable contents of the interfered ions were 50 μg for uranium, 200 μg for zirconium and 500 μg for titanium, respectively. The allowable contents of the familiar ions, such as Ca, Cu and Zn, were all above 10 μg.
Stardand curves of the LREEs and thorium
A series of 5, 10, 15, 20 μg standard LREEs solution and a series of 2, 4, 6, 8 μg standard thorium solution were passed through the column and analyzed, respectively. The linear regression parameters of the LREEs and thorium standard curves were as follows:
Here, A is the absorbance, C is the concentration of the LREEs and thorium and R is the regression coefficient.
Sample analysis
The proposed method was applied to separate and determine the LREEs and thorium in Baotou Iron Ore (East ore, West ore and Main ore). The measured values were compared with the standard values.
It was concluded that the relative deviations (RD) of the measured values and the standard values were 0.32%, 3.13% and 0.27% for the determination of the LREEs, respectively. The relative standard deviations (RSD) were 0.5–1.7% (Table 1).
It was concluded that the relative deviations (RD) of the measured values and the standard values were 0.007%, 0% and 0% for the determination of thorium, respectively. The relative standard deviations (RSD) were 0.66–2.71% (Table 2).
Conclusions
The proposed method was achieved by continuous separation and determination of the LREEs and thorium adsorbed on the micro-column. The eluent of 4 mol L−1 HCl–2 mol L−1 NH4Cl solution can substitute of traditional high concentration hydrochloric acid solution [22] and the volume of the eluent was only a small amount.
The high mass ratio of the LREEs and thorium can be allowable in our study. The breakthrough of thorium did not happen when thorium was eluted with the eluent of the LREEs.
References
Karve, M., Gaur, C.: J. Radioanal. Nucl. Chem. 270, 461 (2006)
Yin, J., Hu, B., He, M., Jiang, Z.: Anal. Chim. Acta 594, 61 (2007)
Mohite, B.S., Jadhav, A.S.: J. Radioanal. Nucl. Chem. 256, 173 (2003)
Luo, M., Li, B., Yang, Z., Liu, W., Sun, Y.: Anal. Sci. 24, 1013 (2008)
Ensafi, A.A., Khayamian, T., Karbasi, M.H.: Anal. Sci. 19, 953 (2003)
Someda, H.H., Sheha, R.R.: Radiochemistry 50, 56 (2008)
Bhattacharyya, A., Mohapatra, P.K., Pathak, P.N., Manchanda, V.K.: J. Radioanal. Nucl. Chem. 268, 323 (2006)
Djingova, R., Ivanova, J.: Talanta 57, 821 (2002)
Gupta, B., Malik, P., Deep, A.: J. Radioanal. Nucl. Chem. 251, 451 (2002)
Mowafy, E.A., Aly, H.F.: J. Radioanal. Nucl. Chem. 250, 199 (2001)
Ansari, S.A., Mohapatra, P.K.: J. Hazard. Mater. 161, 1323 (2009)
Zhang, N., Huang, C., Hu, B.: Anal. Sci. 23, 997 (2007)
Sungur, S.K., Akseli, A.: J. Chromatogr. A 874, 311 (2000)
Shvoeva, O.P., Dedkova, V.P., Savvin, S.B.: J. Anal. Chem. 62, 935 (2007)
Liang, P., Liu, Y., Guo, L.: Spectrochim. Acta B Atom. Spec. 60, 125 (2004)
Tsukada, M., Sato, D., Endo, K., Yanaga, M., Currie, L.A., Glascock, M.D., Ondov, J.M., Han, M.: J. Radioanal. Nucl. Chem. 246, 463 (2000)
Tanaka, K., Takahashi, Y., Shimizu, H.: Anal. Chim. Acta 583, 303 (2007)
Igarashi, K., Akagi, T., Fu, F.F., Yabuki, S.: Anal. Sci. 19, 441 (2003)
Dampare, S.B., Asiedu, D.K., Osae, S., Nyarko, B.J.B., Banoeng-Yakubo, B.: J. Radioanal. Nucl. Chem. 265, 101 (2005)
Dedkova, V.P., Shvoeva, O.P., Savvin, S.B.: J. Anal. Chem. 63, 430 (2008)
Sakamoto, N., Kano, N., Imaizumi, H.: Appl. Geochem. 23, 2955 (2008)
Pinto, D.V.B.S., Martins, A.H.: Hydrometallurgy 60, 99 (2001)
Acknowledgements
The authors are specially thankful to Professor Mrs. Jin-Ru Song for her help with and in support of this work. The project was supported by the National Defense Basic Research Foundation of China (Grant No. A3420060146).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, Gl., Luo, Mb., Xu, Jj. et al. Separation and continuous determination of the light rare earth elements and thorium in Baotou Iron Ore by a micro-column. J Radioanal Nucl Chem 281, 647–651 (2009). https://doi.org/10.1007/s10967-009-0041-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-009-0041-7