Abstract
Properties of polyethylene terephthalate (PET) and polypropylene (PP) can be enhanced by blending both in a specific proportion to get combined superior properties. To make a homogeneous blend, maleic anhydride functionalized PP (PP-g-MAH) was used as a compatibilizer in 60% PET blends. In all blends, PET’s percentage crystallinity was reduced as compared to pure PET because there were hindrances in chains packing because of the presence of PP chains. The addition of compatibilizer in PET and PP blend enhanced interactions resulted in a homogeneous blend with fewer voids. 2.5% of PP-g-MAH was observed to be the optimum value of compatibilizer in the blend of PET and PP. The decrease in free space inside the blend hindered water molecules' passage through the sheets. Owing to this, water vapor permeability of the blend was less compared to pure PET. The addition of the nonpolar PP also influenced the water transmittance rate of blended sheets.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer blends can be defined as mixtures of at least two types of polymers, with physical interactions sometime may also have covalent bonds between them [1]. Among the most widely employed blends generated with improved properties are polypropylene (PP)/Nylon [2], polylactic acid (PLA)/ polybutylene succinate (PBS) [3], ethylene vinyl alcohol (EVOH) /PP [4], EVOH/ polyethylene (PE) [5] and PP/PLA [6] PE/ polyethylene terephthalate (PET) [7]. Polymer blends can be miscible or immiscible. To convert immiscible polymer blend to miscible blend, compatibility between polymers is enhanced by modifying the interphase that will reduce the interfacial energy. The miscibility of the components depends on several factors such as interfacial tension, adhesion between two phases and melt viscosities of the components. Miscibility can be achieved by creating linkages such as, short time cross linking or reversible crosslinking, by introducing ionic interactions or hydrogen bonding. However, the most common method is inclusion or generation of the medium that will improve the interfacial properties in polymer blends, which has been an objective of this research work [8].
Polypropylene (PP) and polyethylene terephthalate (PET) are approved for food contact during storage. PP is stiffer, denser, and more transparent than polyethylene (PE). It has extremely effective barrier properties for water vapor due to its nonpolar nature. PP has low gas barrier properties hence where the high gas barrier is required, such as in food packaging, PP is laminated with aluminum layer [9]. PET has superior gas barrier, glass-like clarity, lightweight and notable aroma barrier property owing to this food packed in PET film sustains its flavor and smell, however, PET has low barrier to water molecule [10]. Researchers are working to improve barrier properties of polymers by blending with a high barrier polymer [11]. The blending of different polymers was reported to be done in single or twin-screw extruder. Permeability can be further reduced by controlling the morphology and to make it homogeneous and well-mixed blend with fewer voids [4, 12,13,14]. A number of compatibilizers were used to enhance the interfacial bonding of PP with polar polymer. Linear low density grafted maleic anhydride (LLDPE-g-MA), polypropylene grafted malic anhydride (PP-g-MA) and hydrogenated styrene butadiene styrene (SBS) block copolymer was used as compatibilizers. This research on the comparison of all the compatibilizers indicated a high efficiency of styrene-ethylene-butylene-styrene grafted malic anhydride (SEBS-g-MA) and PP-g-MA + EPM (ethylene propylene copolymer) but low performance of LLDPE-g-MA [15].
A blend formation is cost-effective as compared to the development of a new homopolymer that has all superior properties or the addition of additives and fillers in the polymer matrix. A miscible blend can provide an optimum barrier, mechanical, thermal and optical properties. A homogeneous blend of PP and PET will provide a combination of required properties for food packaging applications. The research work is focused on the fabrication of PP and PET blends by varying the amount of compatibilizer to check its effect on the morphology, thermal, barrier and viscoelastic properties of blends. PP-g-MAH having up to 2% grafting degree content was used as compatibilizer prepared by reactive extrusion process [16].
Materials and Method
Isotactic polypropylene was purchased from LCY Chemicals CORP with MFI 3.297 g/10 min. The density of PP was 0.908 g/cm3. PP-g-MAH was prepared in the lab by reactive extrusion by following the existing procedure in literature and the highest grafted sample was used [16]. Film grade polyethylene terephthalate (PET) was provided by Gatron Industries limited having MFI 39.36 g/10. The density of the PET was 1.38 g/cm3. All the materials were used as received without further purification.
Preparation of Blends
Blends of PP with PET were prepared by varying composition of PP-g-MAH in an internal mixer. The internal mixer used was Thermo Fisher Scientific HAAKE™ Rheomix Lab Mixer. The internal mixer was first preheated at 270 °C and PET was added at this temperature. After the melting of PET, PP was added in the internal mixer and the temperature was reduced to 230 °C. After 2 min of mixing at 230 °C, PP-g-MAH was added in internal mixer and blended for 7–10 min at 70 rpm. The blend was extracted from internal mixer and cooled at ambient condition. Sheets of all the blends were prepared by compression molding at 200 °C and 2000 psi pressure. 2 mm thickness of the sheet was maintained using a 2 mm spacer (process shown in Fig. 1). Table 1 below shows the composition of all blends where PET is constant in each blend.
Characterization
Fourier Transform Infrared Spectroscopy was done to study the specific interactions in the blend of PP with PET by analyzing the functional groups present in the blend. Generally, FTIR was used in transmittance mode from 4000 cm−1 to 500 cm−1 wavenumber range, at 32 scan numbers at 4 cm−1. To confirm the presence of physical or chemical interaction in the blend of PP and PET, the spectrum was recorded by Bruker Alpha instrument.
Thermal analysis of all blends was done by Perkin Elmer Differential Scanning Calorimeter (DSC) by heating 5–8 mg of the sample from 50 °C to 300 °C at 10 °C per minute scan rate and cooling back with the same rate under N2 atmosphere. The % crystallinity of PET in the blends was calculated by Eq. 1 [17, 18].
where, \({\Delta H}_{f}^{*}\) is the heat of fusion, \({\Delta H}_{c}^{*}\) is the heat of crystallization of PET and \({\Delta H}_{{f}_{100}}\) is the heat of fusion for hypothetically 100% crystalline PET [19]. The value for the enthalpy of fusion of 100% crystalline PET used was 140 J/g [20]. Scanning electron microscopy (SEM) Joel JSM 6490A was performed to study the morphology of blends. Miscibility and fracture behavior of the prepared blends can be analyzed by SEM images.
The water permeability of prepared sheets was determined by ASTM E-96. Sheets were tightly glued on a cup filled with a known weight of water and were placed in humidity and temperature control chamber in the air environment at room temperature and 20% RH. After 24 h. weight of the water inside the cup was measured and evaporated water was calculated. This practice was done for 5 days to estimate the water transmission rate from the sheets. The water vapor transmission rate (WVTR) was calculated by Eq. 2.[21].
where
G = change in water weight
t = time during which change occurs
A = test area
Permeance was measured by Eq. 3.[21]
∆P = vapor pressure difference
Permeability of sheets for water vapors was determined by Eq. 4[21].
Dynamic mechanical analysis (DMA) (TA Instruments) of all blends in comparison of pure PET and PP was done according to ASTM E1640-13 with dual cantilever. DMA was done to study the viscoelastic properties of compatibilized blend in comparison of pure PET and pure PP. The dual cantilever was used owing to the delicate nature of sheets. The sample was run at 5 °C/ min and 1 Hz frequency under nitrogen and test was conducted from -100 °C to 100 °C.
Results and Discussion
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectra of pure PP, pure PET and PET-PP blend are presented in Fig. 2 to study the interactions with these materials. Four adjacent stacks appeared at 2950 cm−1 for the asymmetric stretching of the methyl group (-CH3), at 2915 cm−1 for the asymmetric stretching of –CH2, at 2870 cm−1 for the symmetric stretching of the methyl group (-CH3), at 2815 cm−1 for the symmetric stretching of -CH2-, bending peaks of -CH2- and –CH3 are at 1455 cm−1 and 1370 cm−1 respectively are present in pure PP and in the blend of PET and PP [22, 23] but is not visible in pure PET. In pure PET and blends, peak appeared at 2960 cm−1 and 2900 cm−1 represents the presence of CH2. At 1715 cm-1 peak is for carbonyl group (C = O). A strong peak at 1240 cm−1 which is clear in pure PET and in the blend confirms the presence of ester linkage (C–O–C) [24, 25].
1500–1400 cm−1 is due to the carbon–carbon stretching vibrations in the aromatic ring. In the fingerprint region, there is a peak in PET and blends’ spectra at 1055 cm−1 due to C-O stretch. A much sharp peak at 715 cm−1 in pure PET and blend is of CH bending in benzene ring [26, 27]. As the blend was prepared by physical mixing and no chemical reaction was involved so by comparing the FTIR spectra of the pure PET and pure PP with blend it was clear that no new peak generation and no prominent shift in peaks were observed after blending PET and PP. This confirms the absence of any new bond generation between PET and PP in the blend. Hence the blending is of physical type. MAH-g-PP acted as bridge in generating physical interaction between PP chain and PET molecule because MAH-g-PP contains both polar and nonpolar sites.
Thermal Analysis by Differential Scanning Calorimetry (DSC)
DSC thermogram of blends prepared with and without PP-g-MAH in comparison of pure PET and pure PP is displayed in Fig. 3. From DSC it was deduced that glass transition temperature (Tg) of PET appeared at 82 °C but in the blend, with or without compatibilizer Tg was difficult to detectable, however, the results showed that the Tg was slightly shifted to a lower temperature after processing.
Tm of PP in the blends was increased from 165 °C to 172 °C compare to pure PP due to reason that the addition of PET in PP effected that chains melting behavior. Inside the blends, PET that is in higher concentration started to form a coil-like structure around the PP molecule which is in lower concentration, due to this PP chains demanded high temperature for melting [28].
Tm of PET in the blend was almost the same but latent heat for melting of PET was observed to be decreased due to the formation of coarse crystals at low temperature in blend compare to pure PET. Thermal properties were overall improved by adding compatibilizer as first melting appears at higher temperatures than pure PP and in blend without compatibilizer. There was no effect on the crystallization temperature (Tc) of PET in the compatibilized blends [28, 29].
The heat of fusion of PP in the compatibilized blend was not highly effected but the heat of fusion of PET in the blend was decreased as shown in Fig. 4 and hence crystallinity of PET was low as calculated by Eq. 1. This decrease was because of hindrance created to crystal formation by the interactions generated between PP and PET after adding compatibilizer.
Morphology by Scanning Electron Microscopy (SEM)
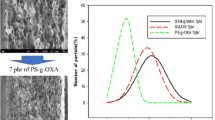
Figure 5 displays the scanning electron microscopy (SEM) images of pure PP and PET and the images of pure PET and pure PP show a completely uniform microstructure. Figure 6 shows the images of compatibilized and uncompatibilized PP and PET blends and from the morphology of the blends, the compatibility between PET and PP phases can be analyzed.
The presence of PP-g-MAH in blends promoted the formation of much finer dispersed morphology, uniformity, and much better adhesion than uncompatibilized blends. This increase in fineness of the blend due to better interaction between blend’s phases resulted in the reduction of voids and hence it reduced the passage of water molecules through the sheets and decrease its permeability for water molecules. Figures 7 and 8 demonstrate the blends by varying the ratio of PP-g-MAH [30,31,32].
In Fig. 7 SEM image of 5%, compatibilizer showed some voids that are probably due to uneven physical interactions between the two polymers. As there was an increase in the amount of compatibilizer more than 2.5%, agglomerates started to appear in some areas in the blend as visible from Fig. 8. PET molecules started to join with each other due to greater interactions between the same types of molecules to stabilize the system [33].
Fracture analysis of blends was also done by SEM images demonstrated in Fig. 9. For fracture analysis, edges of fractured samples are compared to understand phase behavior. The spherical shaped beads belong to PP polymer and the main matrix is PET. These spherical beads are clearly seen to be debonded from the matrix material due to the lack of interfacial adhesion in an uncompatibilized blend. A large number of sumps are clearly visible in the blend without PP-g-MAH owing to the pull out of these weakly adhered polymers. Morphology of compatibilized blends showed a smaller particle size due to greater interaction. Spherically shaped beads are now seemed to be adhered to the matrix by forming bridges. These interactions are due to dipole–dipole attractions between PET’s carbonyl group and maleic anhydride group in PP. In the fractured surface of compatibilized blends the fibrils’ extension can be analyzed as well as plane surface fracture, so it can be illustrated that the blend of PP and PET with PP-g-MAH is moderately ductile [31].
An optimum amount of compatibilizer can provide a homogeneous blend with fewer phase separations. From SEM images, it is clear that 2.5% compatibilizer is the amount of PP-g-MAH that showed the best compatibilizer concentration for PET and PP blend with reduced agglomerations and voids.
Water Vapors Permeability
Water vapors permeability is calculated according to ASTM E-96 and it is displayed in Fig. 10. Diffusion of penetrant molecules depends on the size of molecules, the polarity of material, the temperature on which diffusion occurs, and the concentration difference of molecules across the sheet. Pure PET material has high permeability for water molecules because of its polar nature. By absorbing a high percentage of water molecules, greater diffusion occurs through PET. Figure 10 compares the water vapor permeability of blends with pure PP and PET. From results, it can be deduced that, when PET was blended with PP using 2.5% compatibilizer, its water-resistance improved because of PP matrix that hindered water molecules passage through the sheets. This compatibilized blend resulted in reduction in voids and free space available inside the blend which can allow water molecules to diffuse through the sheet. However, at a higher percentage of compatibilizer, due to uneven physical interactions between the PP and PET voids appeared in the sheets that increased water molecules’ permeability.
In blend without compatibilizer, PET and PP were completely phase-separated that was also analyzed by SEM images. Without the addition of MAH-g-PP in blend, there were no interactions present between PET and PP which created defects in the sheet. When there is less interaction in the blend, sheet was not homogeneous and defective sites allowed the passage of water molecules through the sheet.
It can be illustrated from the above results that 2.5% PP-g-MAH is the optimum value of compatibilizer in the blend which showed less permeability as compare to other blends. Water vapors transmission rate increased in other blends because of no compatibility and less physical interactions between polymers. Hence, it is concluded that the addition of compatibilizer in an optimum amount in the blends improved the water vapor resistance of PET resin in blends.
Dynamic Mechanical Analysis (DMA)
Generally, the multiphase blends of polymer display low mechanical properties because of the lack of interfacial adhesion and weak physical and chemical links between phases. Dynamic mechanical analysis (DMA) is utilized to study the amount of miscibility present between polymers in the blends and its effect on the thermomechanical response. The properties obtained in DMA like storage modulus (G'), loss modulus (G'') and damping (tanδ) of polymer blends usually depend on the polymer structure and degree of crystallinity in the polymer. Figure 11 exhibits the tan delta of PET and PP blends in comparison to pure PP and pure PET.
Pure PET showed a sharp prominent peak at about 85 °C temperature which is the Tg of PET and is almost equal to the data obtained from DSC. Pure PP showed two small shoulders -15 °C and 70 °C temperature. Isotactic polypropylene shows its Tg at -15 °C and chains relaxation occurs at 70 °C. Sometime in semi-crystalline polymers, there is a premelting state between Tg and Tm at which polymer chains display hindered rotation inside the folded crystals. However, PET has a greater area under the peak in comparison of PP so PET has more ability to dissipate energy when load is applied in comparison of pure PP. PP chains showed elasticity in its structure it means it has more ability to store the load than dissipating it. From the compatibilized blends partial miscibility of the components is clear as peak started to merge. The low-temperature peak in all blends started to move towards high-temperature peak.
The behavior is due to the addition of compatbilizer in PET and PP and which allows the components to physically interact and the formation of a partially homogeneous blend. Peak area also increased in all blends in comparison of pure PP which indicated good impact bearing properties in blends [34,35,36]. In blend without PP-g-MAH, the existence of two distinct damping peaks at their original position are representing the components of uncompatibilized blend in the DM spectrum confirming that the blend is incompatible and it shows two-phase morphology.
Figure 12 explains the effect of temperature on the storage modulus of blends in comparison to pure PET and pure PP.
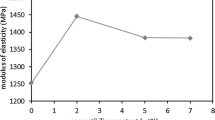
Storage modulus has two relatively flat stages that are joined with a steep portion and in the intermediate portion, softening of the material occurs. Pure PET showed a minimum value of storage modulus and this value was observed to be decrease with temperature regularly. In this temperature range PET in not melted and just soften with increasing temperature because the crystals in PET melt on a long-range of temperature. PP has higher storage modulus than pure PET however PP showed a sudden fall in storage modulus at 0 °C temperature and a high variation in G′ with temperature was noted.
By the addition of PP-g-MAH in the blends, storage modulus was increased in comparison to both pure PET and pure PP. This raise was owing to high interaction and enhanced compatibility between PET and PP. By increasing the amount of compatibilizer storage was not pronounced due to uneven physical interaction that leads to improper phase adhesion. Compatibilizer reduced the transition region in PET and PP blend. It was worth noticing that with the increase in temperature all samples started to achieve the less difference in the value of storage modulus and curves came close because the chains softened. The addition of PP-g-MAH enhanced the storage modulus of blend that leads to high mechanical strength in the blends.[34, 35].
Figure 13 describes the variation in loss modulus with temperature for PET and PP compatibilized blends in comparison to pure PET and pure PP. It shows the energy dissipation measurement of all samples. The value of storage and loss modulus always decreases as less force is required for deforming samples. In all samples initially, it resists molecular segmental motion but with increasing temperature, these kinds of molecular motion are activated. PET has Tg at 82 °C and it is almost that same that was obtained from DSC analysis. Pure PP showed Tg at -15 °C.
By making a compatibilized blend, in C1 Tg of PP was slightly moved at about 10 °C and the Tg of PET was undetectable. It can be deduced that C2 has good compatibility between the PET and PP phases. C3 and C4 also showed an increase in Tg of PP, on the other hand, these two blends also showed the Tg of PET at 90 °C. Samples that have high loss modulus have more force dissipation ability and material is less stiff. It is clear that with increasing temperature, friction for molecular movement reduced, this resulted less energy dissipation and loss modulus continued to be decreased [34, 35].
Conclusion
The summary of this work is, PP-g-MAH can be used as a compatibilizer to make a homogeneous blend of PET and PP with only physical interactions between two polymers. Compatibilized blend showed much better properties in comparison to pure PET and pure PP and blend without compatibilizer. 2.5% content of PP-g-MAH in PET and PP blend with 60% PET ratio offered the best homogeneous blend relative to other concentrations. The addition of PP-g-MAH in blend gave fewer voids, defect-free structure, and high thermal properties. The plastic sheet exhibited an excellent WV barrier for 2.5% PP-g-MAH content. The concentration of compatibilizer greater than 2.5% generated uneven interactions in the phases that eventually allow the passage of water vapors molecules. The thermomechanical properties of compatibilized blends were also higher as compared to pure PP and pure PET.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
Utracki LA (2000) Polymer Blends. vol v. 11. Rapra Technology Limited,
Citterio CSE, Testa G, Bonfatti AM, Seves A (1999) Physico-chemical characterization of compatibilized poly(propylene)/aromatic polyamide blends. Macromol Mater Eng 270(1):22–27
Bhatia A, Bhattacharya GR and Choi HS (2007) Compatibility of biodegradable poly (lactic acid) (PLA) and poly (butylene succinate) (PBS) blends for packaging application. KoreaAustralia Rheology Journal 19 (3):125-131
Faisant AAi-K JB, Bousmina M, and Deschenes L (18 June 1998) Morphology, thermomechanical and barrier properties of polypropylene-ethylene vinyl alcohol blends. Polymer 39 (3):533-545
Torradas JM ZD New laminar oxygen barrier technology for food packaging applications. In: conference proceedings of Society of Plastics Engineers Annual Technical Papers, Montreal, Canada, 1991.
Nalin Ploypetchara PS, Atong D, Pechyen C (2014) Blend of polypropylene/poly(lactic acid) for medical packaging application: physicochemical, thermal, mechanical, and barrier properties. Energy Procedia 56:201–210
Cristina Moniz MDCaMI (2007) Blends of poly(ethylene terephthalate) and low density polyethylene containing aluminium: A material obtained from packaging recycling. J Appl Polym Sci 106(4):2524–2535
Isayev AI (2010) Encyclopedia of Polymer Blends: Volume 1: Fundamentals. John Wiley & Sons,
Tice P (2002) Packaging materials: 3. Polypropylene as a packaging material for foods and beverages, International Life Sciences Institute Europe Belgium
Mangaraj S, Goswami TK, Mahajan P (2009) Applications of Plastic Films for Modified Atmosphere Packaging of Fruits and Vegetables: A Review. Food Engineering Reviews 1:133–158. https://doi.org/10.1007/s12393-009-9007-3
Shorten DW (1982) Polyolefins for food packaging. Food Chem 8(2):109–119
Faisant JBA-KA, Bousmina M, Deschenes L (1998) Morphology, thermomechanical and barrier properties of polypropylene – ethylene vinyl alcohol blends. Polymer 39(3):533–545
Subramanian PM (1985) Permeability barriers by controlled morphology of polymer blends. Polym Eng Sci 25(8):483–487
Dukjoon Kim SWK (2003) Barrier Property and Morphology of Polypropylene/Polyamide Blend Film. Korean J Chem Eng 20(4):776–782
Papadopoulou CP, Kalfoglou NK (2000) Comparison of compatibilizer effectiveness for PET/PP blends: their mechanical, thermal and morphology characterization. Polymer 41(7):2543–2555. https://doi.org/10.1016/S0032-3861(99)00442-5
Shi D, Yang J, Yao Z, Wang Y, Huang H, Jing W, Yin J, Costa G (2001) Functionalization of isotactic polypropylene with maleic anhydride by reactive extrusion: mechanism of melt grafting. Polymer 42(13):5549–5557. https://doi.org/10.1016/S0032-3861(01)00069-6
Sichina W (2000) DSC as problem solving tool: measurement of percent crystallinity of thermoplastics. Perkin Elmer Instruments, and PETech 40
Kong Y, Hay J (2002) The measurement of the crystallinity of polymers by DSC. Polymer 43(14):3873–3878
Oromiehie A, Ebadi-Dehaghani H, Mirbagheri S (2014) Chemical modification of polypropylene by maleic anhydride: Melt grafting, characterization and mechanism. International Journal of Chemical Engineering and Applications 5(2):117
Sichina WJ DSC as Problem Solving Tool: Measurement of Percent Crystallinity of Thermoplastics.
Testing ASf, Materials (2013) Standard test methods for water vapor transmission of materials. ASTM International,
Glaser TK, Plohl O, Vesel A, Ajdnik U, Ulrih NP, Hrnčič MK, Bren U, Fras Zemljič L (2019) Functionalization of polyethylene (PE) and polypropylene (PP) material using chitosan nanoparticles with incorporated resveratrol as potential active packaging. Materials 12(13):2118
Sanbhal N, Li Y, Khatri A, Peerzada M, Wang L (2019) Chitosan Cross-Linked Bio-based Antimicrobial Polypropylene Meshes for Hernia Repair Loaded with Levofloxacin HCl via Cold Oxygen Plasma. Coatings 9(3):168
Chiu H-T, Hsiao Y-K (2006) Compatibilization of Poly(ethylene terephthalate)/Polypropylene Blends with Maleic Anhydride Grafted Polyethylene-Octene Elastomer. J Polym Res 13(2):153–160. https://doi.org/10.1007/s10965-005-9020-z
Mecozzi M (2019) The differentiation of biodegradable and non-biodegradable polyethylene terephthalate (PET) samples by FTIR spectroscopy: A potential support for the structural differentiation of PET in environmental analysis. Infrared Physics & Technology 101. doi:https://doi.org/10.1016/j.infrared.2019.06.008
Espinoza-García K, Marcos-Fernández A, Navarro R, Ramírez-Hernández A, Báez-García JE, Rangel-Porras G (2019) Polymerization of ε-caprolactone with degraded PET for its functionalization. J Polym Res 26(8):180. https://doi.org/10.1007/s10965-019-1821-6
Seki Y (2019) Enhancement of Electrical Conductivity of Polyethylene Terephthalate (PET) Fabrics via Ionic Liquids. Polymer-Plastics Technology and Materials 58(1):70–76. https://doi.org/10.1080/03602559.2018.1466163
Song Q, Xia Y, Hu S, Zhao J, Zhang G (2016) Tuning the crystallinity and degradability of PCL by organocatalytic copolymerization with δ-hexalactone. Polymer 102:248–255
Guo C, Zhou L, Lv J (2013) Effects of expandable graphite and modified ammonium polyphosphate on the flame-retardant and mechanical properties of wood flour-polypropylene composites. Polym Polym Compos 21(7):449
Abdul Razak NC, Inuwa IM, Hassan A, Samsudin SA (2013) Effects of compatibilizers on mechanical properties of PET/PP blend. Compos Interfaces 20(7):507–515. https://doi.org/10.1080/15685543.2013.811176
Oyman ZO, Tinçer T (2003) Melt blending of poly(ethylene terephthalate) with polypropylene in the presence of silane coupling agent. J Appl Polym Sci 89(4):1039–1048. https://doi.org/10.1002/app.12228
Xanthos M, Young MW, Biesenberger JA (1990) Polypropylene/polyethylene terephthalate blends compatibilized through functionalization. Polym Eng Sci 30(6):355–365. https://doi.org/10.1002/pen.760300607
Akbari M, Zadhoush A, Haghighat M (2007) PET/PP blending by using PP-g-MA synthesized by solid phase. J Appl Polym Sci 104(6):3986–3993
Thirtha V, Lehman R, Nosker T (2006) Morphological effects on glass transition behavior in selected immiscible blends of amorphous and semicrystalline polymers. Polymer 47(15):5392–5401
Serhatkulu T, Erman B, Bahar I, Fakirov S, Evstatiev M, Sapundjieva D (1995) Dynamic mechanical study of amorphous phases in poly (ethylene terephthalate)/nylon-6 blends. Polymer
Zdrazilova N, Hausnerova B, Kitano T, Saha P. (2004). Rheological behaviour of PP/PET and modified PP/PET blends. II. Dynamic viscoelastic properties Polymers and Polymer Composites. 12(5):433–448
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tariq, A., Afzal, A., Rashid, I.A. et al. Study of thermal, morphological, barrier and viscoelastic properties of PP grafted with maleic anhydride (PP-g-MAH) and PET blends . J Polym Res 27, 309 (2020). https://doi.org/10.1007/s10965-020-02291-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02291-2