Abstract
Mechanically robust and self-healing epoxy composites are highly desired to satisfy the increasing demand of high-performance smart materials. Herein, a dual functionalized epoxy composite (EpF-MWCNT-PA-BM) with self-healing performance based on Diels-Alder chemistry has been investigated. The furfuryl grafted epoxy (EpF) and furfuryl modified MWCNTs (MWCNT-F) are reacted with bifunctional maleimide (BM) and normal anhydride curing agent (PA) to form a covalently bonded and reversibly crosslinked epoxy composite with two types of intermonomer linkage. That is, thermally reversible Diels-alder bonds between the furan groups of both epoxy and MWNCTs with malemide and thermally stable bonds of epoxide and anhydride groups. MWCNTs act as both reinforcer and a healant in the epoxy composite. In this way, the cured epoxy composite possessed not only enhanced mechanical properties but also thermal remendability that enabled elimination of cracks. The latter function took effect as a result of successive retro-DA and DA reactions, which led to crack healing upto 79.82% healing efficiency in a controlled manner through chain reconnection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biomimetic materials are attracting considerable amount of attention, owing to their ability to mimic the structural, mechanical, and biological properties of natural tissues. The ability to repair themselves in response to damage and recover material structures and functions after failure events is an essential property of living systems; the transfer of this property to synthetic materials becomes a major topic of research in materials science during the recent years [1,2,3].
The chance to attain the self-healing feature as an intrinsic property of the synthetic materials aroused great appeal. The dynamic epoxy resin would provide new possibilities for high performance materials and applications. Owing to its excellent thermal stability and solvent resistance, remarkable adhesive strength, and ease of curing and processing, epoxy resin has long been considered one of the most extensively applied thermosetting polymers in a wide range of fields, including potting materials for electronics, coatings and adhesives, printed circuit boards, etc. [1, 4,5,6,7,8].
Several technologies have emerged over the past several years for the self-healing of epoxy-based systems, most notably being polymers containing microcapsules [7, 9,10,11,12], vascular systems [13, 14], and dynamic covalent bonds [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31] and so on. The self-healing properties of microcapsule-based composites were obtained by incorporating microcapsules containing reactive chemicals into the epoxy matrix. Upon damage on formation of cracks, the microcapsules can release its chemicals and recover the defect. However, through this method, the polymer systems can be healed for only once which limits its application. Moreover, the synthetic process involved in vascular systems is too complex to produce in mass. In an alternative approach, self-healing is obtained by the modification of polymers with functional groups based on dynamic bonds.
The dynamic bond, which can unlock the cross-linked network under external stimuli and subsequently lock to again form the crosslinked network can impart the property of self-healing of resins. The processes such as ester exchange [15,16,17], disulfide exchange [18,19,20], and the Diels-Alder (DA) bond [21,22,23,24,25,26,27,28,29,30,31] are generally the dynamic bonds used for reversible network formation. Among all dynamic bonds based healing systems to date, DA bonds are supposed to be one of the most reliable systems which result in highly efficient reversibility and moderate sensitivity to temperature [21, 32, 33]. The process involves monomers carrying diene or dienophile groups are reacted to yield a cross-linked polymer. Healing of cracks can be achieved by heating the polymer to a temperature required for a reversible DA reaction. The heat leads to a partial disconnection of the polymer chains and enhances the mobility of individual chains. Upon cooling, the chains are crosslinked again by formation of new DA bonds [34,35,36,37]. Wudl et al. [22, 23] reported the self-healing of polymeric networks by utilizing the thermo-reversible covalent bond nature of the DA reaction between the furfuryl and maleimide groups. Some researchers followed the Wudl’s approach and studied thermo-healable epoxy resin by modifying the epoxy monomer with the DA group. Liu and Hsieh used the epoxy compounds as precursors to synthesize thermally mendable cross-linked polymers from multifunctional maleimide and furan compounds [24]. Subsequently, Tian et al. synthesized epoxy resin with furan groups and reacted with bifunctional maleimide to form a crosslinked epoxy that can heal the cracks resulting from r-DA and DA reactions [25, 26]. Ober and co-workers developed a series of reworkable epoxy compounds that incorporated thermally cleavable groups, such as secondary or tertiary esters [38, 39]. These groups were chosen to decompose between a temperature of 200 and 300 °C so that the cured epoxy can be recovered without damaging the underlying structure. Also, Small et al. made a thermally-removable encapsulant for an epoxy system consisting of bis(maleimide) compound and tris(furan) or tetrakis(furan), which can be easily removed by heating to temperatures higher than 90 °C, preferably in a polar solvent [40]. However, undesirable side reactions between maleimides and free amines may occur during the cross-linking process. Kuang et al. synthesized a new diamine DA adduct cross-linker to cure the common epoxy monomers, but the procedure is too complex to avoid the side-reactions [29]. Subsequently, Turkenburg et al. developed a two-step process to minimize the side-reactions: the first step is the prepolymerization of furfuryl amine with epoxy monomers, and then the subsequent cross-linking with BM to obtain epoxy resin with self-healing property [30].
Development of cured epichlorohydrins having properties at par with cured conventional epoxy resin has already been studied earlier implying that the synthesized epoxy materials might be applicable for the field in which conventional epoxy resin is employed [25, 41]. Moreover, carbon nanomaterials, which show good compatibility with polymeric materials due to their large π-conjugated system, have been widely used as efficient fillers in fabrication of mechanical enhanced self-healing polymer materials because of their ultrahigh mechanical strength [42,43,44,45,46,47]. Despite these advances, to the best of our knowledge, few reports are available until now about the use of MWCNT as functional nanofiller for high-performance self-healing epoxy adhesive. Keeping that in mind, herein we have reported a mechanically robust self-healing epoxy composite with MWCNTs having enhanced mechanical and self-healing properties by a covalently and reversibly bonded nanofiller strategy based on DA bonding. Bismaleimide (BM) can react with furan rings of both MWCNTs and epoxy to yield cured DA-epoxy-MWCNT composites. Among different BM, 1,1′-(Methylenedi-4,1-phenylene)bismaleimide was chosen for preparation of DA-epoxy-MWCNT composites because it had relatively low melting point, which is in favor of the mixing, processing, and reconstruction of DA network. Also, the epoxide groups of EpF can react with a traditional curing agent like anhydride to form an epoxy network, providing the material with outstanding mechanical properties as usual. As illustrated in Scheme 1, the furfuryl modified epoxy (EpF) and furfuryl functionalized MWCNT (MWCNT-F) were reacted with bismaleimide (BM) via DA reaction to form a covalently bonded and reversibly cross-linked MWCNT/epoxy composite. The furfuryl functionalized MWCNT is expected to play dual roles of reinforcer and a kind of healant in the epoxy composites.
Experimental section
Materials used
N,N,N/,N//,N// Pentamethyldiethylenetriamine (PMDETA) (99%, Aldrich), Ethyl α-Bromo isobutyrate (EBrB) (98%, Aldrich), α-bromoisobutyryl bromide (98%, Aldrich), Copper bromide (CuBr) (98%, Aldrich), MWCNT (REDEX tech. pvt. Ltd.), Furfuryl amine (99%, Aldrich), Furfurylmethacrylate (FMA) (98%, Merck), N,N/−(4,4/−diphenylmethane) bismaleimide (BM) (99%, Merck), Phthalic anhydride (PA) (99%, Merck), Epichlorohydrine (99%, Alfa-aesar) Sodium hydroxide (NaOH) (Alfa-aesar) were used as received. Solvents ethyl acetate, hexane, Tetrahydrfuran (THF) and Dimethylformamide (DMF) were supplied from Merck India and dried using the standard methods.
Preparation of Furfuryl-functionalized MWCNT (MWCNT-F) by Atom Transfer Radical Polymerization (ATRP)
MWCNT-F was synthesized by ATRP process using initiator functionalized MWCNT (MWCNT-Br). MWCNT-Br was synthesized by a previously reported process [47]. In a typical procedure, a 100 mL dried round bottom flask containing MWCNT-Br (15 mg) and PMDETA (32.8 mg, 0.19 mmol) was degassed and refilled with nitrogen three times. FMA (5.4 g, 32.5 mmol) and dichloromethane (4 mL) were added, and the reaction mixture was degassed again. After stirring for 1 h at room temperature, CuBr (15.1 mg, 0.105 mmol) was added, and the flask was placed in a thermostated oil bath at 110 °C. After 3 min EBrB (16.7 mg, 0.1 mmol) was injected. After 14 h the polymerization was stopped, the reaction mixture was then poured into methanol to extract the polymer sample.
Synthesis of furan functionalized epoxy, N,N-diglycidyl-furfurylamine (EpF)
Furan functionalized epoxy, EpF was synthesized by a previously reported procedure [25]. Typically, epichlorohydrine (8.5 mL, 0.1 mol) was charged into a 250 mL three-necked round-bottom flask equipped with a stirrer, a thermometer, and a nitrogen inlet. The solution was then heated to 40 °C and furfuryl amine (5.22 g, 0.05 mol) was added dropwise while keeping the solution temperature below 60 °C. The reaction mixture was stirred under N2 atmosphere for about 5 h at 60 °C and then cooled down to room temperature. 8.75 mL of aqueous sodium hydroxide solution (50% (w/v)) was then added dropwise to the reaction mixture within 1 h. The reaction was allowed to proceed for additional 5 h at 30 °C. After some time, the organic layer was collected using ethyl acetate, washed with water for several times and dried with anhydrous sodium sulfate, filtrated, and evaporated to give a 80% liquid. The product was further purified by passing it through a silica gel column using mixed solvent of ethyl acetate/hexane (1/3). The solvent was removed on a rotary evaporator, and lastly, a light yellow colour liquid was obtained with a yield of 50%.
Preparation of EpF-MWCNT nanocomposites by DA adduct formation
To achieve the EpF-MWCNT nanocomposites by DA reaction with BM, a solution method was chosen. Firstly, BM (5.71 g, 0.016 mol) was dissolved in anhydrous THF (50 mL). The solution was charged into a 100 mL three-necked round-bottom flask equipped with magnetic stirring. Then, EpF (6.68 g, 0.032 mol) was slowly added into the solution. Predetermined amount of synthesized MWCNTs containing fufuryl moiety (5 wt%) were added into the polymer solution. The solution was refluxed under N2 atmosphere at about 60 °C for 24 h and cooled down to room temperature. The reaction solution was poured into a large excess of diethyl ether to get the precipitate. The precipitate was separated, washed with methanol, and then dried under vacuum at room temperature.
Preparation of EpF-MWCNT-BM-PA crosslinked polymer
First, 17.13 g (0.048 mol) BM was dissolved in 20 g (0.096 mol) EpF under stirring at 90 °C. About 5 wt% of furfuryl containing MWCNT dissolved in minimum amount of THF were then added onto it and stirred for about for 15 min. Then, 25.86 g (0.1536 mol) PA was mixed with the above mixture at 80 °C and stirred for an additional 15 min. The resultant homogeneous liquid was degassed, poured into a mold, and cured at 70 °C for 24 h. The curing temperature was selected depending on the optimal temperature for DA bond formation (see the sub-section in the Results and discussions section: DA and retro-DA reactions between EpF-MWCNT and BM), so that most furan and maleimide groups could take part in the reaction prior to the solidification of the system. Furthermore, a non-stoichiometric ratio of epoxy ring/anhydride of 1:0.8 was used to slow down curing of the resin.
Solvent exposure test
Typically, 0.1 g of the crosslinked resin was added into a solvent in a closed flask and kept at room temperature under static conditions. After 1 day, samples were taken out and the mass of the residue was determined. The swelling ratio was calculated as
where W is the weight of EpF-MWCNT-PA-BM before its addition into the solvent and Wf is the weight of EpF-MWCNT-PA-BM after immersion without drying.
Self-healing study
A razor blade was used to make cuts on the crosslinked polymer film (5 mm thick) so as to generate visible cracks. The samples were then thermally treated at different temperatures (depending on type of simulation tests described in Results and discussions) and annealed at 70 °C for different times. Images of cracks on the surface of sample before and after healing were obtained with an optical microscope and SEM.
Instruments and methods
1H NMR was measured by JEOL 400 MHz NMR instrument using CDCl3 as solvent. FTIR spectra of the samples were recorded with a Nicolet Impact-410 IR spectrometer (USA) in KBr medium at room temperature in the range of 4000–400 cm−1. DSC was performed Thermogravimetric analyses (TGA) was studied in a Shimadzu TA50 thermal analyzer under nitrogen atmosphere at a heating rate of 5 °C/min in the range of 30–600 °C. Molecular weights and the polydispersity were measured by a gel permeation chromatography (GPC) instrument equipped with a Waters Styragel column (HR series 3, 4E) with THF as eluent at a flow rate of 0.7 mL/min. Optical microscopic images of the microcapsules was taken using Polarizing Microscope BA310 Pol. To evaluate self-healing ability of the materials, the method proposed by Jones et al. was employed to evaluate the self-healing ability of the polymers [48]. Healing efficiency, He is defined as:
Tensile properties were examined using Universal Testing Machine (UTM, Zwick, Z010) at ambient temperature. A loading rate of 10 mm/min was applied for the test. Razor blade prenotched samples were first broken to failure, giving the fracture toughness, σvirgin and immediately clamped together to heal. Healed samples were tested again to measure the regained fracture toughness, σhealed. condition.
Results and discussions
Thermal reversibility of EpF-MWCNT-BM crosslinked polymer
The primary objective of this work is to develop a thermally remendable epoxy/MWCNTs nanocomposite by inducing thermally reversible groups into the cured networks. The MWCNTs were incorporated onto the EpF matrix by DA reaction of furfuryl groups of MWCNT-F with the help of a bismaleimide crosslinker. A higher temperature for DA reactions were used to increase the reaction efficiency and to decrease the time. Accordingly the temperature was set to 70 °C and the DA reaction was carried out for almost 16 h. First, DA reaction of the furan and maleimide groups in an equivalent molar mixture of EpF and MWCNT-F with BM was monitored by both FTIR and 1H-NMR spectroscopy. As shown in Fig. 1a, a specific peak at 1771 cm−1 (designated by red line) was observed which is a characteristic absorption peak standing for DA addition product. In addition, the absorption peak at 737 cm−1 of the EpF-MWCNT-BM, which was assigned to the furan groups becomes much weaker due to the DA reaction of the furan groups of MWCNT-F as well as EpF with BM.
Then, 1H-NMR was also carried out at specific time intervals to monitor the DA reaction proceeding in the mixture solution at 70 °C. The results in Fig. 2 show that at the beginning of the reaction, no characteristic peaks between 5.1 and 5.3 ppm corresponding to the DA adduct are observed (Fig. 2a). After 6 h, these peaks appear (Fig. 2b), and become more evident when the reaction has proceeded for 16 h (Fig. 2c). The feasibility of the DA reaction is thus confirmed [49, 50].
Moreover, DSC was also performed to further confirm the formation of DA bond in the composite as illustrated in Fig. 3a. The Tg peak can be observed at 71.09 °C, and when the temperature is increased to above 100 °C, a broad endothermic peak corresponding to the r-DA reaction of the composite can be observed followed by two exothermal peaks at 180 and 230 °C due to the epoxide ring-opening reaction and polymerization of maleimide moieties. Furthermore, the incorporation of MWCNTs onto the epoxy matrix by DA reversible reactions was also confirmed by the DSC results. The DSC of the simulation test of EpF crosslinked by BM shows a Tg at 68.3 °C which is approximately 3° lower than the Tg of EpF-MWCNT-BM. The reason may be attributed to the strong and favorable interaction of EpF with the MWCNT-F via reversible DA reaction. However, it should be noted that not a very large (3°) increase in Tg was observed in this case as traditionally obtained, mainly because of the attachment of the aliphatic chains in the form of poly(furfurylmethacrylate) onto the surface of MWCNTs. Furthermore a recovery experiment of EpF-MWCNT-BM crosslinked resin was carried out at 120 °C using DMF as the solvent. The r-DA reaction occurs and leads to removal of MWCNT-F from the EpF matrix. After cooling to room temperature and being precipitated in excess water, r-DA product was obtained and further characterized by GPC. The GPC trace after removing MWCNT-F and BM (Fig. 3b) suggested that the r-DA product had a relatively large PDI with a bimodal distribution with peak values recorded at 2.1–2.8 × 103 and 0.32–0.38 × 103 g/mol respectively. This phenomenon could be attributed to the release of BM, whose molecular weight is 358.35 g/mol, from de-cross-linked resins together with EpF after r-DA reaction. Therefore, the Mn of r-DA product was much smaller than that of EpF. These results confirmed that r-DA reaction did occur, and, as a consequence, the EpF, MWCNT-F and BM were released in the DMF.
Additionally, the retro-DA reaction of the DA adduct EpF-MWCNT-BM crosslinked polymer was evaluated by 1H NMR (Fig. 4). It was observed that the peaks at 5.1–5.3 ppm ascribed to the DA adduct (Fig. 4a) entirely disappear in the spectrum of the retro-DA product (Fig. 4b). These data demonstrate that the retro-DA reaction can be completed at 120 °C for 40 min [49, 50].
Thermal reversibility of EpF-MWCNT-BM-PA crosslinked polymer
The curing reaction of furfuryl group containing EpF and MWCNT-F with PA and BM was also carried out at 70 °C for 24 h. In the FTIR spectrum of cured EpF-MWCNT-BM-PA (Fig. 5), the corresponding peaks of the epoxide groups at 918 cm−1 (oxirane ring breathing) and 851 cm−1 (C-O-C) disappear, indicating that all the epoxide groups have reacted with the anhydride groups of PA to form epoxy networks. A broad peak at 3500 cm−1 attributed to the hydroxyl groups emerges which is quite evidently a result of this reaction. Besides, the DA reaction of furan and maleimide groups present in EpF as well as MWCNT-F in the cured sample is also proved by the existence of the peak at 1753 cm−1.
As mentioned in the Introduction, the cured EpF-MWCNT-BM-PA polymer consist of two types of crosslinked covalent bonds: (a) thermally stable bonds from the reaction between the epoxide and anhydride groups, and (b) thermally reversible bonds from the DA reaction of the furan and maleic groups of both EpF and MWCNTs. The DA and retro-DA reactions between EpF and BM, MWCNT-F and BM and also EpF-MWCNT-BM have been proven by the simulation tests (Fig. S1-S7 in ESI), however whether these stable epoxy networks would inhibit the thermally reversible reactions is still not clear [47, 51,52,53]. Therefore, the thermal reversibility of the cured EpF-MWCNT-BM-PA composite polymer should be studied since it is related to thermal remendability of the material directly.
Figure 6 shows the heating DSC curves of the cured EpF-MWCNT-BM-PA crosslinked polymer. The first heating curve (Fig. 6a) shows an endothermic peak at about 140 °C, while the second heating curve (Fig. 6b) shows no endothermic peak. It is thus reasonable to deduce that the endotherm on the first curve must be an outcome from the retro-DA reaction. The second curve shows no endothermic peak because there is not enough time for the furan and maleimide recovered moieties to be reconnected during the successive cooling and reheating processes, thus no more retro-DA reaction occurs in such short span of time. In comparison to the retro-DA reaction temperature of 120 °C in EpF-MWCNT-BM (Fig. 3), retro-DA reaction temperature in anhydride cured EpF-MWCNT-BM-PA (140 °C) is higher which indicates the restraining effect of the cured epoxy matrix. It can also be said that the furan and maleimide groups reconnection in the cured EpF-MWCNT-BM-PA matrix proceed at a much slower rate than that in EpF-MWCNT-BM, due to the same restriction of the epoxy network after its curing by PA. Nevertheless, from the DSC results it can be concluded that the retro-DA reaction pathway is still accessible under heating conditions and is preferred over the bond-breaking degradation reaction in the cured epoxy network.
The reversibility of the crosslinked EpF-MWCNT polymer composite was further studied by using cyclic retro-DA (heat treatment at 140 °C for 40 min to attain a retro-DA sample) and DA (heat treatment at 70 °C for 24 h to attain a DA sample) reactions and monitored by DSC shown in Fig. 7. The higher heating temperature of the DA reaction was chosen just to increase the reaction efficiency. The original cured EpF-MWCNT-BM-PA crosslinked resin (DA0) gives an endothermic peak of the retro-DA reaction at 140 °C, while the rDA1 only shows a transition at 130.5 °C. This implies that the retro-DA reaction has already been completed during the heat treatment at 140 °C for 40 min. Subsequently, the cleavage groups of rDA1 are allowed to reconnect by the DA reaction giving DA1 and on DSC, an endothermic peak appears again at 145 °C. It is evident that the DA reaction between the disconnected furan and maleimide moieties has taken place definitely during the thermal treatment process. The recyclability of retro-DA and DA reactions in the crosslinked EpF-MWCNT-BM-PA polymer has been verified by the repeated endotherms in this manner for the DA and retro-DA samples.
DSC heating traces of EpF-MWCNT-BM-PA crosslinked polymer (heating rate: 5 °C/min). DA0: as-synthesized sample; rDA1: DA0 treated at 140 °C for 40 min, and then normally cooled; DA1: rDA1 treated at 70 °C for 16 h, and then cooled; rDA2: DA1 treated at 142 °C for 20 min, and then cooled to room temperature; DA2: rDA2 treated at 70 °C for 24 h, and then cooled; rDA3: DA2 treated at 144 °C for 20 min, and then cooled to room temperature; DA3: rDA3 treated at 70 °C for 48 h, and then cooled
The characteristic parameters of the reactions observed by DSC (Fig. 7) are summarized in Table 1. It should be noted that the endothermic peak temperature of the DA reaction slightly increases with heating cycles, implying that the retro-DA reaction becomes more difficult. This phenomenon may be due to the increase in crosslink density of the epoxy resin after heat treatment. However, the DA and retro-DA reactions in the cured EpF-MWCNT-BM-PA polymer can still be considered as reversible.
Solvent exposure tests and crosslinking density
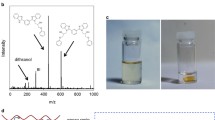
Solvent exposure tests were carried out to evaluate the cross-link density of EpF-MWCNT-BM-PA crosslinked polymer composite. A swelling ratio of 140.6 was calculated which clearly indicates the high crosslinking density of the polymer composite. Figure 8 shows the solubility of crosslinked EpF-MWCNT-BM as well as EpF-MWCNT-BM-PA composite and its rDA products in DMF. The crosslinked DA adduct of EpF-MWCNT-BM was found to be insoluble in DMF due to the presence of reversible DA bonds as shown in Fig. 8a. However when thermally treated at 120 °C for about 30 min, the rDA product was obtained which becomes soluble in DMF (Fig. 8b), indicating that the cross-linked network was disconnected by rDA reaction. In case of EpF- MWCNT -BM-PA crosslinked polymer, as expected it remains insoluble in DMF at room temperature (Fig. 8c). However when heated to 140 °C partial solubility was observed (Fig. 8d). The reason is due to presence of two types of bonds: thermally stable bonds from the reaction between epoxide and anhydride which does not break down after heating to 140 °C and thus attributing to non-solubility part of the polymer and thermally reversible bonds formed between furan groups of MWCNT-F and EpF with BM which leads to the solubility of the crosslinked polymer in DMF. Due to uncrosslinking of the crosslinked polymer to its respective furan and maleimide moieties, the polymer became partly soluble in DMF with the crosslinking due to anhydride with epoxide groups remaining intact. This further justifies the accessibility of reversible DA and rDA in the crosslinked polymer.
Self-healing and mechanical properties
The thermal induced self-healing properties of EpF-MWCNT-BM-PA crosslinked polymer was systematically investigated using an optical microscope equipped with a hot stage source. First, a large cut was made on the bonded crosslinked sample using a razor blade and immediately clamped together under thermal treatment. Since the crack interfaces have a higher refractive index, they are easily visually monitored. Figure 9 showed the optical microscope photographs of the injured samples before and after self-healing and treated at different temperatures. The whole crack could be healed within 40 min at 140 °C. The proposed mechanism for the self-healing process is like this, incorporation of BM into the polymer matrix and the subsequent cross-linking leads to increase in hardness and decrease in mobility. When the knife-cut sample is then heated to 140 °C, it induces rDA reaction and decrosslinking of the network. This increases the chain mobility leading to reflow of the materials towards the crack site and when the temperature was then lowered, full healing occurs due to reformation of the bonds via DA reaction.
Optical microscopy images to observe the thermal remendability of the cured EpF-MWCNT-BM-PA nanocomposite polymer. The damaged samples were firstly treated at (a) 100 °C, (b)120 °C and (c) 140 °C for 40 min, respectively. Next, they were moved to an oven preset at 70 °C for (i) 0 h, (ii) 8 h and (iii) 16 h, respectively
Moreover the thermal induced self-healing was also monitored by SEM analysis shown in Fig. 10. The images shows the surface morphology of a crack created by a sharp razor blade before and after healing (for 16 h at 140 °C). It is very clear from the figure that a successful healing of the crack occurred.
The tensile properties of the EpF-MWCNT-BM-PA crosslinked polymer were measured at room temperature (Table 2). The cured version of the newly synthesized epoxy-MWCNT composite (EpF-MWCNT-BM-PA) has moderately lower strength as compared to the cured bisphenol-A epoxy based MWCNT composites. Also it has a comparatively higher toughness without sacrificing its tensile strength. The main reason may be attributed to the presence of two crosslinking linkages consisting of reversible DA and stable anhydride-epoxy bonds. Due to reversibility of the DA bonds, it not only contributes to the self-healing behaviour but also manifests considerable toughness to the system. It may also be noted that EpF-MWCNT-BM-PA has a much higher strength as compared to EpF-BM-PA due to the incorporation of MWCNTs which is a well known strength enhancer [56, 57].
Based on the reversibility by DA/rDA reactions, the epoxy composites exhibited thermal healing capability. The tensile properties of EpF-MWCNT-BM-PA crosslinked resin before and after self-healing was evaluated to illustrate the healing efficiency. A good recovery of morphology and tensile strength were observed. Figure 11a shows the tensile strength of tested samples before and after healing with respect to time required for DA reaction. The samples were thermally treated to 140 °C for about 40 min and the temperature was then lowered down to allow the DA reaction to occur. As shown in Fig. 11b, the tensile strength of the original resin was 67.9 MPa; the injured one decreased to 5.0 MPa, where the testing sample fractured precisely at the scratch trace. After the thermal-induced healing process at 140 °C and without compression stress, the tensile strength of the healed one could recover to 54.3 MPa, and thus, the healing efficiency was 79.82%.
In a control experiment the self-healing of the epoxy resin without MWCNTs i.e. EpF-BM was also carried out and the results are shown in Fig. 12. The crack could be healed at 120 °C within 30 min followed by treatment at 60 °C in an oven for 16 h. The self-healing efficiency was found to be 67% (Fig. 12b) which is lesser than the healing efficiency of EpF-MWCNT-BM-PA. Increasing the healing temperature even upto 150 °C has no affect on the healing efficiency of EpF-BM. The reason may be attributed to the fact that the reinforcement of MWCNTs on EpF not only enhances the mechanical properties but also helps in dissipation of heat throughout the polymer nanocomposite matrix surface to give a better healing performance which coincides with the results obtained by Li et al. [58].
Also pristine MWCNTs surface can also act as dienophile for furfuryl functionalized epoxy, EpF. Thus in a comparative experiment, DA reaction between MWCNTs (0.045 g) and EpF (0.45 g, 0.002 mol) was carried out in NMP (90 mL) for 48 h at room temperature under open atmosphere [59]. The FTIR spectra in Fig. 13A clearly shows the peaks at 1600–1440 cm−1 diminishes which is assigned to the C=C groups in aromatic rings of MWCNTs. Appearance of the bands at 3020 cm−1 (C-H in oxirane ring), 1250 and 853 cm−1 (C-O-C), and 920 cm−1 (oxirane ring breathing) indicates that the MWCNT was functionalized by EpF through DA reaction. Also a peak at around 1782 cm−1 appears which is a characteristic absorption peak for DA reaction. This indicates that DA reaction is taking place in between pristine MWCNTs and EpF. When PA is added, the corresponding peaks of the epoxide groups at 920 cm−1 (oxirane ring breathing) and 853 cm−1 (C-O-C) disappear and a broad peak at 3500 cm−1 attributed to the hydroxyl groups appears indicating that all the epoxide groups have reacted with the anhydride groups of PA to form epoxy networks with the peak at 1782 cm−1 remaining intact. On heating to 160 °C the peaks at 1782 cm−1 (C=C in DA adduct) diminishes and the the peaks at 1600–1440 cm−1 (C=C in MWCNTs) and 742 cm−1 (furan groups of EpF) emerges clearly indicating the progress of rDA reaction. Thermal remendability of artificial damage on the nanocomposite having pristine MWCNTs also takes place by reversible DA-rDa reaction as confirmed by optical micrograph image in Fig. 13B. It was observed that although self-healing starts at 140 °C but proper healing takes place only when temperature was raised to 150–160 °C. The reason of higher temperature requirement for pristine MWCNTs as compared to its functionalized counterpart may be due to the lower ring strain in MWCNTs for it to be directly used as a dienophile [60]. Moreover, well controlled thickness of the MWCNTs is possible by using MWCNT-F because of its functionalization by poly(furfurylmethacrylate) through the ATRP ‘grafting from’ approach [47].
The healing efficiency shown in Fig. 11 is only attributed to the first healing cycle of the epoxy composite. The DA reaction being reversible, rearrangement of the network structure would take place by proceeding cyclic DA/rDA reactions. Figure 14a shows the variation in strength of the healed composites upto four healing cycles. The injured sample was treated at 140 °C to regain the healed sample and again the sample was injured at the same position to determine the recovered tensile strength of the composite. The results clearly demonstrate that a reasonable amount of strength of the composite can be recovered even after three successive injuries with the healing efficiency being 79.82%, 67.91% and 51.80% for the first, second and third healing cycles. The healing efficiency decreases considerably after fourth healing cycle leading to only 28.52%. Thus, the epoxy-MWCNT composites enjoy the merits of self-healing without losing integrity and load bearing ability, which is quite outstanding among the self-healing epoxy composites with a very good self-healing efficiency and repeatable healing property.
Conclusions
A novel strategy for fabricating recyclable and thermally repairable epoxy composite EpF- MWCNT-BM-PA is well-established by the incorporation of MWCNTs into the epoxy by dynamic covalent DA bonds. Cured EpF-MWCNT-BM-PA consisted of two types of linkages, one thermally reversible DA bonds from the reaction between furan and maleimide groups and second, thermally stable bonds from the reaction between epoxide and anhydride groups. The results of FTIR, NMR, DSC, and GPC analysis and the solvent-exposure test confirmed the dynamic performance of DA networks. Additionally, thermal reversibility of the crosslinked matrix was also studied upto four healing cycles and considerably good healing efficiency of 79.82%, 67.91% and 51.80% was determined for the first three healing tests. We anticipate this high performance self-healing epoxy composites might provide strategy for the fabrication of smart materials with potential applications in electronic and engineering fields.
References
White SR, Sottos NR, Geubelle PH, Moore JS, Kessler MR, Sriram SR, Brown EN, Viswanathan S (2001) Autonomic healing of polymer composites. Nature 409:794–797
Ghosh B, Urban MW (2009) Self-repairing oxetane-substituted chitosan polyurethane networks. Science 323:1458–1460
Cordier P, Tournilhac F, Soulie-Ziakovic C, Leibler L (2008) Self-healing and thermoreversible rubber from supramolecular assembly. Nature 451:977–980
Pathak AK, Garg H, Singh M, Yokozeki T, Dhakate SR (2019) Enhanced interfacial properties of graphene oxide incorporated carbon fiber reinforced epoxy nanocomposite: a systematic thermal properties investigation. J Polym Res 26:23
Haase MF, Grigoriev DO, Mohwald H, Shchukin DG (2012) Development of nanoparticle stabilized polymer Nanocontainers with high content of the encapsulated active agent and their application in water-borne anticorrosive coatings. Adv Mater 24:2429–2435
Wu G, An JL, Tang XZ, Yang JL, Xiang YA (2014) A versatile approach towards multifunctional robust microcapsules with tunable, restorable, and solvent-proof Superhydrophobicity for self-healing and self-cleaning coatings. Adv Funct Mater 24:6751–6761
Yuan YC, Ye XJ, Rong MZ, Zhang MQ, Yang GC, Zhao JQ (2011) Self-healing epoxy composite with heat-resistant healant. ACS Appl Mater Interfaces 3:4487–4495
Tang XL, Zhou Y, Peng M (2016) Green preparation of epoxy/graphene oxide nanocomposites using a glycidylamine epoxy resin as the surface modifier and phase transfer agent of graphene oxide. ACS Appl Mater Interfaces 8:1854–1866
Jin HH, Mangun CL, Stradley DS, Moore JS, Sottos NR, White SR (2012) Self-healing thermoset using encapsulated epoxy-amine healing chemistry. Polymer 53:581–587
Hillewaere XKD, Teixeira RFA, Nguyen LT, Ramos JA, Rahier H, Du Prez FE (2014) Autonomous self-healing of epoxy thermosets with thiol-isocyanate chemistry. Adv Funct Mater 24:5575–5583
Gao L, He J, Hu J, Wang C (2015) Photoresponsive self-healing polymer composite with photoabsorbing hybrid microcapsules. ACS Appl Mater Interfaces 7:25546–25552
Guo W, Jia Y, Tian K, Xu Z, Jiao J, Li R, Wu Y, Cao L, Wang H (2016) UV-triggered self-healing of a single robust SiO2 microcapsule based on cationic polymerization for potential application in aerospace coatings. ACS Appl Mater Interfaces 8:21046–21054
Luterbacher R, Trask RS, Bond IP (2016) Static and fatigue tensile properties of cross-ply laminates containing vascules for self-healing applications. Smart Mater Struct 25:015003
Hart KR, Sottos NR, White SR (2015) Repeatable self-healing of an epoxy matrix using imidazole initiated polymerization. Polymer 67:174–184
Ghazi A, Ghasemi E, Mahdavian M, Ramezanzadeh B, Rostami M (2015) The application of benzimidazole and zinc cations intercalated sodium montmorillonite as smart ion exchange inhibiting pigments in the epoxy ester coating. Corros Sci 94:207–217
Altuna FI, Pettarin V, Williams RJJ (2013) Self-healable polymer networks based on the cross-linking of epoxidised soybean oil by an aqueous citric acid solution. Green Chem 15:3360–3366
Long R, Qi HJ, Dunn ML (2013) Modeling the mechanics of covalently adaptable polymer networks with temperature-dependent bond exchange reactions. Soft Matter 9:4083–4096
Lafont U, Van Zeijl H, Van der Zwaag S (2012) Influence of cross-linkers on the cohesive and adhesive self-healing ability of polysulfide-based thermosets. ACS Appl Mater Interfaces 4:6280–6288
Hernandez M, Grande AM, Dierkes W, Bijleveld J, Van der Zwaag S, Garcia SJ (2016) Turning vulcanized natural rubber into a self-healing polymer: effect of the disulfide/polysulfide ratio. ACS Sustain Chem Eng 4:5776–5784
Lei ZQ, Xiang HP, Yuan YJ, Rong MZ, Zhang MQ (2014) Room-temperature self-healable and remoldable cross-linked polymer based on the dynamic exchange of disulfide bonds. Chem Mater 26:2038–2046
Nguyen LTT, Nguyen HT, Truong TT (2015) Thermally mendable material based on a furyl-telechelic semicrystalline polymer and a maleimide crosslinker. J Polym Res 22:186
Chen XX, Dam MA, Ono K, Mal A, Shen HB, Nutt SR, Sheran K, Wudl FA (2002) A thermally re-mendable cross-linked polymeric material. Science 295:1698–1702
Chen XX, Wudl F, Mal AK, Shen HB, Nutt SR (2003) New thermally remendable highly cross-linked polymeric materials. Macromolecules 36:1802–1807
Liu YL, Hsieh CY (2006) Crosslinked epoxy materials exhibiting thermal remendablility and removability from multifunctional maleimide and furan compounds. J Polym Sci Part A: Polym Chem 44:905–913
Tian Q, Yuan YC, Rong MZ, Zhang MQ (2009) A thermally remendable epoxy resin. J Mater Chem 19:1289–1296
Tian Q, Rong MZ, Zhang MQ, Yuan YC (2010) Synthesis and characterization of epoxy with improved thermal remendability based on Diels-Alder reaction. Polym Int 59:1339–1345
Bai N, Simon GP, Saito K (2013) Investigation of the thermal self-healing mechanism in a cross-linked epoxy system. RSC Adv 3:20699–20707
Bai N, Saito K, Simon GP (2013) Synthesis of a diamine cross-linker containing Diels–Alder adducts to produce self-healing thermosetting epoxy polymer from a widely used epoxy monomer. Polym Chem 4:724–730
Kuang X, Liu GM, Dong X, Liu XXG, Xu JJ, Wang DJ (2015) Facile fabrication of fast recyclable and multiple self-healing epoxy materials through diels-alder adduct cross-linker. J Polym Sci Part A: Polym Chem 53:2094–2103
Turkenburg DH, Fischer HR (2015) Diels-Alder based, thermo-reversible cross-linked epoxies for use in self-healing composites. Polymer 79:187–194
Guo YK, Li H, Zhao PX, Wang XF, Astruc D, Shuai MB (2017) Thermo-reversible MWCNTs/epoxy polymer for use in self-healing and recyclable epoxy adhesive. Chin J Polym Sci 35:728–738
Rivero G, Nguyen LTT, Hillewaere XK, Du Prez FE (2014) One-pot thermo-remendable shape memory polyurethanes. Macromolecules 47:2010–2018
Heo Y, Sodano HA (2014) Self-healing polyurethanes with shape recovery. Adv Funct Mater 24:5261–5268
Zeng C, Seino H, Ren J, Hatanaka K, Yoshie N (2013) Bio-based furan polymers with self-healing ability. Macromolecules 46:1794–1802
Barthel MJ, Rudolph T, Teichler A, Paulus RM, Vitz J, Hoeppener S, Schubert US (2013) Self-healing materials via reversible crosslinking of poly (ethylene oxide)-block-poly (furfuryl glycidyl ether)(peo-b-pfge) block copolymer films. Adv Funct Mater 23:4921–4932
Liu YL, Chen YW (2007) Thermally-reversible cross-linked polyamides with high toughness and self-repairing ability from maleimide- and furan-functionalized aromatic polyamides. Macromol Chem Phys 208:224–232
Zhang JJ, Niu Y, Huang CL, Xiao LP, Chen ZT, Yang KK, Wang YZ (2012) Self-healable and recyclable triple-shape PPDO-PTMEG co-network constructed through Thermoreversible Diels-Alder reaction. Polym Chem 3:1390–1393
Yang S, Chen JS, Korner H, Breiner T, Ober CK (1998) Reworkable epoxies: thermosets with thermally cleavable groups for controlled network breakdown. Chem Mater 10:1475–1482
Chen JS, Ober CK, Poliks MD (2002) Characterization of thermally reworkable thermosets: materials for environmentally friendly processing and reuse. Polymer 43:131–139
Small JH, Loy DA, Wheeler DR, McElhanon JR, Saunders RS, Method of making thermally removable polymeric encapsulants. U.S. Patent No. 6,271,335. 7 Aug. 2001
Shen X, Liu X, Wang J, Dai J, Zhu J (2017) Synthesis of an epoxy monomer from bio-based 2, 5-Furandimethanol and its toughening via Diels–Alder reaction. Ind Eng Chem Res 56:8508–8516
Byrne MT, Gun’ko YK (2010) Recent advances in research on carbon nanotube-polymer composites. Adv Mater 22:1672–1688
Schwenke AM, Hoeppener S, Ulrich SS, Schubert US (2015) Synthesis and modification of carbon nanomaterials utilizing microwave heating. Adv Mater 27:4113–4141
Kuang X, Liu GM, Dong X, Liu XG, Xu JJ, Wang DJ (2015) Facile fabrication of fast recyclable and multiple self-healing epoxy materials through diels-alder adduct cross-linker. Polym Sci Part A: Polym Chem 53:2094–2103
Li JH, Zhang GP, Deng LB, Zhao SF, Gao YJ, Jiang K, Sun R, Wong CP (2014) In situ polymerization of mechanically reinforced, thermally healable graphene oxide/polyurethane composites based on Diels–Alder chemistry. J Mater Chem A 2:20642–20649
Saikia BJ, Dolui SK (2015) Preparation and characterization of an azide–alkyne cycloaddition based self-healing system via a semiencapsulation method. RSC Adv 5:2480–92489
Kong H, Gao C, Yan D (2004) Controlled functionalization of multiwalled carbon nanotubes by in situ atom transfer radical polymerization. J Am Chem Soc 126:412–413
Jones AS, Rule JD, Moore JS, White SR, Sottos NR (2006) Catalyst morphology and dissolution kinetics of self-healing polymers. Chem Mater 18:1312–1317
Chen X, Dam MA, Ono K, Mal AK, Shen H, Nutt SR, Wudl F (2002) A thermally re-mendable cross-linked polymeric material. Science 295:1698–1702
Pratama PA, Sharifi M, Peterson AM, Palmese GR (2013) Room temperature self-healing thermoset based on the Diels–Alder reaction. ACS Appl Mater Interfaces 5:12425–12431
Zhang HB, Lin GD, Zhou ZH, Dong X, Chen T (2002) Raman spectra of MWCNTs and MWCNT-based H2-adsorbing system. Carbon 40:2429–2436
Antunes EF, Lobo AO, Corat EJ, Trava-Airoldi VJ, Martin AA, Veríssimo C (2006) Comparative study of first-and second-order Raman spectra of MWCNT at visible and infrared laser excitation. Carbon 44:2202–2211
Chang CM, Liu YL (2009) Functionalization of multi-walled carbon nanotubes with furan and maleimide compounds through Diels–Alder cycloaddition. Carbon 47:3041–3049
Gojny FH, Wichmann MHG, Köpke U, Fiedler B, Schulte K (2004) Carbon nanotube-reinforced epoxy-composites: enhanced stiffness and fracture toughness at low nanotube content. Compos Sci Technol 64:2363–2371
Abdalla M, Dean D, Robinson P, Nyairo E (2008) Cure behavior of epoxy/MWCNT nanocomposites: the effect of nanotube surface modification. Polymer 49:3310–3317
Sadek EM, El-Nashar DE, Ward AA, Ahmed SM (2018) Study on the properties of multi-walled carbon nanotubes reinforced poly (vinyl alcohol) composites. J Polym Res 25:249
Kuang X, Liu G, Dong X, Wang D (2016) Enhancement of mechanical and self-healing performance in multiwall carbon nanotube/rubber composites via Diels–Alder bonding. Macromol Mater Eng 301:535–541
Li QT, Jiang MJ, Wu G, Chen L, Chen SC, Cao YX, Wang YZ (2017) Photothermal conversion triggered precisely targeted healing of epoxy resin based on thermoreversible diels–alder network and amino-functionalized carbon nanotubes. ACS Appl Mater Interfaces 9:20797–20807
Pramanik NB, Singha NK (2015) Direct functionalization of multi-walled carbon nanotubes (MWCNTs) via grafting of poly (furfuryl methacrylate) using Diels–Alder “click chemistry” and its thermoreversibility. RSC Adv 5:94321–94327
Hirsch A, Backes C (2010) Carbon nanotube science. Synthesis, properties and applications. By Peter JF Harris. Angew Chem Int Ed 49:1722–1723
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1691 kb)
Rights and permissions
About this article
Cite this article
Handique, J., Dolui, S.K. A thermally remendable multiwalled carbon nanotube/epoxy composites via Diels-Alder bonding. J Polym Res 26, 163 (2019). https://doi.org/10.1007/s10965-019-1804-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1804-7