Abstract
α,ω-Hydroxy telechelic poly(ε-caprolactone) (HOPCLOH) was synthesized by ring-opening polymerization (ROP) of ε-caprolactone (ε-CL).The ROP was catalyzed by ammonium decamolybdate in the presence of ether diols [HO-(CH2-CH2-O)m-H] (where m = 2, 3, 4, 5, 6, and 8) as initiators. The homopolymer HOPCLOH was obtained with the ether group (EG) [HO-PCL-(CH2-CH2-O)m-PCL-OH (HOPCLOH)] as part of the backbone of the polyester with a systematic increase in the segment of the EG. The number average molecular weight (Mn) for all samples were similar in the range of oligomers (Mn = 1240–1510 Da) to have a significant effect of the EG. The effect of the EG on the physical properties was evaluated by differential scanning calorimetry (DSC) where the crystallinity of HOPCLOH and the size of the EG showed a relationship inversely proportional. Poly(ester-urethanes) (PEUs) derived from HOPCLOH exhibited an elastomeric behavior, where long chains of EG induced poor mechanical properties. The use and selection of the ether diols as initiators in the ROP of CL to synthesize HOPCLOH was not trivial because these EG substituents affected the crystallinity, and the mechanical properties of their PEUs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In polymer chemistry, a homopolymer is described as “a polymer containing only one type of repeat unit” [1], a case is the poly(ε-caprolactone) (PCL), a linear aliphatic biodegradable polyester with an ester group and five methylenes as repetitive unit [2, 3] and used as biomaterial in tissue engineering applications [4]. Generally, PCL is synthesized by ring-opening polymerization (ROP) of the ε-caprolactone (CL) using a metallic catalyst and an initiator [2,3,4,5,6,7,8]. Usually, the initiators are alcohols (R-OH) [2,3,4,5,6,7,8], and diols (HO-R-OH) [9,10,11], obtaining an α-hydroxy PCL (R-PCL-OH or PCLOH) [2,3,4,5,6,7,8], and α,ω-hydroxy telechelic PCL (HO-PCL-R-PCL-OH or HOPCLOH) [9,10,11], respectively. Recently, the effect of aliphatic initiators as substituents in the polyesters such as PCL [3, 12], poly(L-lactide) (PLLA) [13, 14] and poly(glycolide) (PGA) [15] has been reported.
The topic on the structure-property in the PCL oligomers [3, 12, 16, 17] and their diblock copolymers [17] has been studied in the last decade. In this sense, HOPCLOH [9,10,11] is one of the most interesting architectures in the PCL derivatives because HOPCLOH is the precursor of triblock copolymers [18,19,20] and poly(ester-urethanes) (PEUs) [21,22,23,24,25,26,27,28,29,30]. Some initiators used in the synthesis of HOPCLOH are derived from aliphatic or ether groups, for example, ethylene glycol [HO(CH2)2OH] [23], 1,8-octanediol [HO(CH2)8OH] [11], diethyelene glycol [HO(CH2)2O(CH2)2OH] [24,25,26], etc. The chemical nature of HOPCLOH (HO-PCL-R-PCL-OH or HO-R-PCL-OH) involve that the aliphatic or ether group (R) from the initiator is inserted in the main chain of the aliphatic polyester [where R = −(CH2)2- [23], −(CH2)8- [12], −(CH2)2O(CH2)2- [24,25,26], etc.].
The motivation of this research is to understand the effect of an ether group [CH2CH2O]m inserted in the main chain of HOPCLOH and its effect on the physical properties of the aliphatic polyester. Ether diols HO[CH2CH2O]mH are very common reagents used in polymer synthesis such as polyesters [24,25,26] and polyurethanes [30,31,32]. Additionally, the similarities between the ether groups and polyethylene glycol (PEG) can provide knowledge on the frontier between homopolymers and triblock copolymers derived from the PCL.
In this work is presented the synthesis, characterization, and comparison of HOPCLOH homopolymers [HOPCL(CH2CH2O)mPCLOH, where m = 2, 3, 4, 5, 6, and 8] (Scheme 1). The goal of this systematic study is to analyze the similarities and differences regarding structure-property of HOPCLOH with varied sizes of the ether groups (EG). Additionally, in a second step PEUs derived from HOPCLOH macrodiols and 1,6-hexamethylene diisocyanate (HDI) were prepared and characterized by FT-IR, NMR, DSC, and mechanical properties.
Experimental section
Materials
Diethylene glycol, triethylene glycol, tetraethylene glycol, pentaethylene glycol, hexaethylene glycol, octaethylene glycol, ε-caprolactone (CL), 1,2-dichloroethane (DCE), 1,6-hexamethylene diisocyanate (HDI) and tin(II) 2-ethylhexanoate [Sn(Oct)2] were purchased from Aldrich Chemical Co. Ammonium heptamolybdate tetrahydrate (NH4)6[Mo7O24].4H2O (Hep) (Fluka) was ground with a pestle and mortar before use.
A typical procedure for the synthesis of α,ω-hydroxy telechelic poly(ε-caprolactone) (HOPCL4OH or macrodiol) by ammonium decamolybdate as catalyst and diethylene glycol (DEG) [HO(CH2CH2O)2H] as initiator
Polymerization was performed in absent of solvent (bulk polymerization) in a dried 25 ml round-bottom flask. Ammonium heptamolybdate tetrahydrate [(NH4)6[Mo7O24].4H2O (Hep), 3 mg], ε-caprolactone (CL) (50 mmol), 5.707 g), and diethylene glycol (DEG) (5 mmol, 530 mg) were charged and heated to reflux by stirring them in an oil bath at 150 °C for 30 min (molar ratio CL/Hep = 20,600 and CL/DEG = 10). Ammonium decamolybdate (NH4)8[Mo10O34] was obtained in situ in the solid state by thermal decomposition of ammonium heptamolybdate [(NH4)6[Mo7O24] [33]. The product synthesized was analyzed without purification. Number average molecular weight (Mn) and conversion were monitored by 1H NMR. After reaction time, an aliquot of crude of the reaction was dissolved in CDCl3 and derivatized with two drops of trifluoroacetic anhydride (TFAA) to prevent overlapping between methylene attached to hydroxyl and ethylene glycol groups and analyzed by 1H NMR. In 1H NMR spectrum, the peaks at 2.36 [-CH2-CO-O-, Ipol, repetitive unit], 3.82 [F3C-CO-O-CH2-CH2-O-CH2-CH2-O-CO-, Ieg, monosubstitution of diethylene glycol] and 3.76 [-CO-O-CH2-CH2-O-CH2-CH2-O-CO-, Ieg, bisubstitution of diethylene glycol] were used to quantify the Mn in two steps: (1) degree of polymerization (DP). DP(NMR) = Ipol/#Hpol ÷ Ieg/#Heg. Ipol and Ieg represent the integrals of the methylenes obtained by 1H NMR from the polyester [-CH2-CO-O-] and diethylene glycol group [F3C-CO-O-CH2-CH2-O-CH2-CH2-O-CO- and -CO-O-CH2-CH2-O-CH2-CH2-O-CO-] peaks, respectively, #Hpol and #Heg represent the number of protons that contributed to the peaks. Finally, the equation is DP(NMR) = Ipol/2 ÷ Ieg/4. (2) The number-average molecular weight (Mn). Mn(NMR) = (MW(CL)).(DP(NMR)) + MW(diol), where MW is the molecular weight of the repetitive unit (CL), and diol (diethylene glycol), respectively; DP(NMR) was previously calculated in step 1. Mn(calcd) = 1250, Mn(NMR) = 1300 (Conv. = 99%), Mn(GPC) = 2720, Mw/Mn = 1.32. IR(cm−1) 3446 (ν, OH, PCL), 2942 (νas, CH2, PCL), 2865 (νs, CH2, PCL), 1721 (ν, C=O, PCL), 1470 (δs, CH2, PCL), 1162 (νas, C-(C=O)-O, PCL), 1044 (νas, O-C-C, PCL), 732 (ρ, CH2, PCL). NMR data for HOPCL4OH. 1H NMR after derivatization with TFAA (400 MHz, CDCl3, ppm): δ 4.50 [F3C-CO-O-CH2-CH2-O-, DEG monosubstitution and unreacted DEG], 4.35 [-CO-CH2-CH2-CH2-CH2-CH2-O-CO-CF3, PCL], 4.27 [-CO-O-CH2-CH2-O-CH2-CH2-O-CO-, DEG bisubstitution and F3C-CO-O-CH2-CH2-O-CH2-CH2-O-CO-, DEG monosubstitution], 4.10 [(-CO-CH2-CH2-CH2-CH2-CH2-O-)n, PCL], 3.82 [F3C-CO-O-CH2-CH2-O-, DEG monosubstitution and unreacted DEG], 3.76 [-CO-O-CH2-CH2-O-CH2-CH2-O-CO- DEG bisubstitution and F3C-CO-O-CH2-CH2-O-CH2-CH2-O-CO-, DEG monosubstitution], 2.35 [(-CO-CH2-CH2-CH2-CH2-CH2-O-)n, PCL], 1.77 [-CO-CH2-CH2-CH2-CH2-CH2-O-CO-CF3, PCL], 1.66 [(-CO-CH2-CH2-CH2-CH2-CH2-O-)n, PCL], 1.38 [(-CO-CH2-CH2-CH2-CH2-CH2-O-)n, PCL]. Note: in the case of samples with DP(calcd) = 20 a molar ratio CL/ether diol = 20 was used.
Synthesis of poly(ester-urethane) (PEU) derived from HOPCL4OH and 1,6-hexamethylene diisocyanate (HDI)
The reaction was carried out in a 25 ml round bottom flask previously dried. 2.34 g of HOPCL4OH [Mn(NMR) = 1300] was charged [according to 1H NMR analysis, it is assumed that 8% of unreacted diol (HO-R-OH, in this particular case diethylene glycol (DEG)) is present in the polymer sample, so this fraction was considered in the preparation, Mn = 1200 (1.951 mmol)], and then 1,6-hexamethylene diisocyanate (HDI) (2.224 mmol, 374 mg) and tin(II) 2-ethylhexanoate [Sn(Oct)2] (34 mg, ~ 3 drops) were added as diisocyanate and catalyst, respectively, and dissolved in 8 ml of 1,2-dichloroethane (DCE). A molar ratio 1:1.14 (HOPCL4OH: HDI) with a slight excess of HDI was used to prevent the reaction of moisture present in the HOPCL4OH. After 1 h of reaction at 80 °C, a fresh portion of solvent was added (~ 1 ml) to prevent a high viscosity. Then, the reaction mixture was stirred for another 2 h at 80 °C. The PEU4 film was obtained by casting in a leveled Teflon surface within a fume cupboard. The cast solution (at 80 °C) was covered with a conical funnel to protect it from dust and to avoid an excessively fast solvent evaporation an allowed to stand a room temperature for 12 h. Next, the PEU film was released and dried under vacuum. Using the same methodology, other eight different PEUs were synthesized. Mn(GPC) = 246,330, Mw/Mn = 1.72. IR (cm−1) 3327 (ν, N-H, urethane), 2939 (νas, CH2, PCL), 2864 (νs, CH2, PCL), 1721 (ν, C=O, PCL), 1684 (ν, C=O, urethane), 1468 (δs, CH2, PCL), 1160 (νas, C-(C=O)-O, PCL), 1044 (νas, O-C-C, PCL), 732 (ρ, CH2, PCL). In all samples of PEUs, the band at 2250 cm−1 detected by FT-IR and attributed to diisocyanate group in the HDI was absent.
Characterization methods
Nuclear Magnetic Resonance (NMR). 1H and 13C NMR were recorded at room temperature on a Varian Inova or Mercury 400 MHz (400 MHz 1H and 100 MHz 13C). CDCl3 was used as solvent and all spectra were referenced to the residual solvent CDCl3 [δ (ppm) 7.26 (1H) and 77.0 (13C)]. Fourier Transform Infrared Spectroscopy (FT-IR). Homopolymers (HOPCLOHs), and poly(ester-urethanes) (PEUs) films were recorded with attenuated total reflectance spectroscopy (ATR) accessory in a Perkin-Elmer Spectrum One FT-IR spectrometer. Differential Scanning Calorimetry (DSC). Thermograms were performed in a Mettler Toledo DSC822e instrument. Three scans were obtained with two heating (25–100 °C and − 100 - 100 °C) and one cooling (100 - -100 °C) between them, at a rate of 10 °C/min and under a nitrogen purge. Gel permeation chromatography (GPC). (a) The case for HOPCLOH: GPC measurements were determined using a Waters gel permeation chromatograph equipped with a Waters 1515 isocratic high-performance liquid chromatography (HPLC) pump and Waters 2414 refractive index (RI) detector. A set of three Waters columns conditioned at 35 °C were used to elute samples at the flow rate of 1 mL/min HPLC grade tetrahydrofuran (THF). Polystyrene standards (Polymer Laboratories) were used for calibration. (b) The case for PEU: GPC measurements were recorded by a PerkinElmer gel permeation chromatograph (Series 200 LC pump) equipped with a refractive index detector (IR 200a). A set of ResiPore columns (Polymer Laboratories) conditioned at 70 °C were used to elute samples at the flow rate of 0.3 mL/min of HPLC-grade N, N-dimethylformamide (DMF) with LiBr (0.1 wt.%). Polystyrene standards (Polymer Laboratories) were used for calibration. Mechanical properties. The mechanical properties were measured in an MTS testing machine equipped with a 100 N load cell. Type 3 dumbbell test pieces (according to ISO 37) were cut from the films. A crosshead speed of 200 mm/min was used. The strain was measured from crosshead separation and referred to 12 mm initial length. At least three samples were evaluated for each PEU.
Results and discussion
α,ω-Hydroxy telechelic poly(ε-caprolactone) (HOPCLOH) using ether diols as initiators
A family of linear aliphatic polyesters derived from α,ω-hydroxy telechelic poly(ε-caprolactone) (HOPCLOH) were synthesized by ring-opening polymerization (ROP) of ε-caprolactone (CL) in the present of different ether diols as initiators [HO(CH2CH2O)mH, where m = 2, 3, 4, 5, 6, and 8], the purpose in the preparation of these species is to understand the effect of the linear ether group (EG) substituents on the physical properties (crystallinity and melting point) of HOPCLOH and eventually, their poly(ester-urethanes) (PEUs) (subsection 3.2). For example, the ROP of CL was realized by bulk polymerization in the presence of diethylene glycol [HO(CH2CH2O)2H] and (NH4)8[Mo10O34] as an initiator and catalyst, respectively, after 30 min at 150 °C, a high conversion (99%) was quantified by 1H NMR (Table 1). To have a high contribution of the linear ether group in the HOPCLOH, the range in the values of the degree of polymerization (DP) and number average molecular weight (Mn) were 10 and 1240–1550 Da., respectively. A good concordance between Mn(calcd) and Mn(NMR) for all HOPCLOH samples was observed, this involved a control on the polymerization with a gradual increase of the content of the EG (from 4 to 16 methylenes) in the main chain of HOPCLOH from 8 to 24 wt.%; however, Mn(calcd) and Mn(GPC) showed significant differences (Table 1, last column) that were attributed to the overestimation of Mn determined by GPC for HOPCLOH which is relatively common due to polystyrene standards used in the calibration curve. Additionally, polydispersity was moderate (1.27–1.47). For the synthesis of poly(ester-urethanes) (PEUs) in the second subsection (3.2) the Mn(NMR) was used in our calculations.
All species derived from HOPCLOHs are homopolymers because these were obtained from ether diols (monodisperse organic molecule) as initiators in the ROP of CL. On the contrary, in the synthesis of a triblock copolymer such as poly(ε-caprolactone)-b-polyethylene glycol-b-poly(ε-caprolactone) (PCL-b-PEG-b-PCL), it is synthesized from polyethylene glycol (PEG) as macroinitiator (polydisperse organic macromolecule). So, the molecular weight distribution in a triblock (or also diblock) copolymer is more complex than homopolymer, this evidence can be demonstrated by MALDI-TOF spectrometry, and it has previously been reported [17, 34, 35].
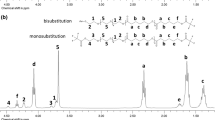
To visualize the repetitive unit and end groups in the HOPCLOH all samples where derivatized by trifluoroacetic anhydride (TFAA) to prevent the overlapping of peaks between methylene attached to the hydroxyl [-CH2OH] and methylene from the ether groups [-OCH2CH2OCH2CH2O-], and eventually, generating a trifluoroacetate ester groups [-CH2O(C=O)CF3] [36]. In Fig. 1, the 1H NMR spectra for three different samples of HOPCLOH are showed, where the number of methylenes presents in the ether groups were increased as part of PCL main chain. For example, in the case of HOPCL2OH signals of the methylenes derived from diethylene glycol previously used as initiator are visualized now as part of PCL backbone in two modes a) monosubstitution [H-O-CH2(b)-CH2(a)-O-CH2(a)-CH2(b)-O-PCL] and b) bisubstitution [PCL-O-CH2(b)-CH2(a)-O-CH2(a)-CH2(b)-O-PCL]. Additionally, in all HOPCLOH samples, a fraction of unreacted diol is estimated, in this sense, in a previous study, during the ROP of CL in the presence of ethylene glycol as an initiator, a fraction of unreacted ethylene glycol was quantified in approximately in 8% on the crude product [12]. In general, FT-IR spectra for HOPCLOH samples shows six characteristics bands at 3446 (ν, -OH), 2942 (νas, -CH2-), 2865 (νs, -CH2-), 1721 (ν, C=O), 1162 (νas, -C-(C=O)-O-), and 732 (ρ, -CH2-) cm−1.
1H NMR (400 MHz) spectra in CDCl3 at room temperature for a HOPCL4OH, b HOPCL6OH, and c HOPCL8OH (Table 1)
The effects of different ether group (EG) substituents [(CH2CH2O)n] in the HOPCLOH were analyzed regarding their thermal properties using scanning differential calorimetric (DSC). DSC analysis for all HOPCLOH samples is presented in Table 2, where a systematic increase in the number of methylenes [(CH2CH2O)n] is visualized from 4 (HOPCL4OH) to 16 (HOPCL16OH). In the same manner, the weight percent of an ether group (EG %) was increased from HOPCL4OH (8%) to HOPCL16OH (24%) and its effect on the glass transition temperature (Tg = −67 - -70 °C) was negligible (Fig. 2). However, the effect of the EG % on the enthalpy of fusion (ΔHm) and crystallinity (xPCL) was evidenced with a systematic decrease, for example, xPCL from 48% (HOPCL4OH) to 38% (HOPCL16OH) (Fig. 3). The melting temperature (Tm) exhibited a double peak for all HOPCLOH samples (Fig. 2), this profile can be interpreted as two different sizes of crystallites or crystallites in two different environments, crystallites more embedded in amorphous domains (low Tm1) and others in zones of high crystallinity (high Tm2). In a previous publication [12], the effect of alkyl group (AG) diols [HO(CH2CH2)mOH] used as initiators in the ROP of CL to synthesize HOPCLOH produced a single endothermic peak (Tm) in all samples with a relative high values of crystallinity (xi) respect to the results in this work. In Fig. 4 these differences are illustrated, where a large gap between the HOPCLOH derived from AG and EG is showed, so, the use and selection of the AG or EG diols to prepare HOPCLOH is not a trivial decision because the macrodiol produced can decrease (EG) or increase (AG) its crystallinity in terms of their substituents. This effect can be explained due to the chemical nature of the EG [HO(CH2CH2O)mH] vs AG [HO(CH2CH2)mOH] diols used as initiators. The main contrast between two substituents is the oxygen atom in the repetitive unit. In polymers the oxygen improve the mobility in the main chain, and that is the reason that two linear polymers such as polyethylene (PE) and polyethylene glycol (PEG) are very different in terms of their crystallinity values, usually PE present high crystallinity respect to PEG at the same Mn. Complementary, the Tm of PE (130 °C) is higher than PEG (66 °C) [37]. In our case, all diols derived from EG even with 16 carbons are liquids at room temperature (r.t.), in contrast, AG diols from 10 to 16 carbons were solids at r.t. In this work, the Tm of HOPCLOH derived from EG has two peaks (31–37 and 39–44 °C) but one of them is low in comparison with Tm of HOPCLOH derived from AG where a single peak (41–46 °C) was observed. So, the EG disrupt the crystalline domains of PCL. In conclusion, the EG as substituents in the HOPCLOH decrease the crystallinity and Tm, this is fundamentally opposed to the HOPCLOH derived from the AG.
Effect of substituents on the crystallinity in the oligomers HOPCLOH using alkyl (Mn = 1370–1550, [12]) or ether diols (Mn = 1240–1510, this work) as initiators in the ROP of CL
Complementary, the physical properties according to the melting point (Tm) and crystallinity (xPCL) of HOPCLOH are similar to a triblock copolymer of PCL-b-PEG-b-PCL with low Mn (≤ 400 Da) of PEG segment and where the Tm mainly was contributed by PCL crystalline domains [38,39,40]. The similarities are consistent because in both cases of HOPCLOH (this work) and PCL-b-PEG-b-PCL [40] their ether diols initiators (HO[CH2CH2O]mH) or macroinitiators (HOPEGOH) used to synthesize them are liquids at room temperature, and the unique semicrystalline polymer under this temperature was the PCL segment.
Poly(ester-urethanes) (PEUs)
Chemical species as the HOPCLOH represent a precursor of poly(ester-urethanes) (PEUs) for its reaction with diisocyanates (OCN-R-NCO). A series of PEUs derivatives from HOPCLOH with EG in the main chain were synthesized. Typically, HOPCL4OH and 1,6-hexamethylene diisocyanate (HDI) were reacted under the presence of tin(II) 2-ethylhexanoate [Sn(Oct)2] as a catalyst in 1,2-dichloroethane (DCE) as solvent at 80 °C for 3 h. All PEUs samples were prepared under similar methodology, but with a variation in the number of methylenes [(-CH2CH2O-)m, where m = 2, 3, 4, 5, 6 and 8] of the EG in the HOPCLOH. Under the previous procedure, eight different PEUs with high Mn (Mn(GPC) = 187,880–246,330 Da) and moderate polydispersity (Mw/Mn = 1.72–2.06) were obtained (Table 3). To corroborate the functional groups in the PEUs the analysis by FT-IR showed characteristics bands at 3325, 1722, 1684, and 1536 cm−1 attributed to the vibrations of N-H (urethane, ν), C=O (ester, ν), C=O (urethane, ν), and N-H (urethane, δ), respectively. The band at 2250 cm−1 attributed to diisocyanate group from HDI was absent, indicating its reaction to produce PEUs. Complementary, in Fig. 5 1H NMR spectrum for PEU4 illustrated the classic peak at 3.12 ppm assigned to the methylene attached to urethane group (-CH2-NH-(C=O)-O-), and the absent of methylenes close to hydroxyl groups [-CH2OH, δ 3.64] confirmed the urethane functional group in the main chain. A similar pattern was evidenced for the rest of the PEUs samples.
1H NMR (400 MHz) spectra in CDCl3 at room temperature for PEU4 (Table 3)
The thermal properties for PEUs are illustrated in Table 3, in where two transitions were visualized such as Tg (–52–55 °C) and Tm (25–30 °C) (Fig. 6). If the values of Tg and Tm from PEUs are compared respect to their HOPCLOH precursors is evident that the Tg increase and the Tm decreased (Fig. 7), this is because the urethane group induces a physical crosslinking by hydrogen bonding between the PEUs chains involving an increase of rigidity causing the increase of the Tg. Complementary, due to the intermolecular bonding of the urethane groups, it provides a random disruption in the order of PCL chains and consequently, a reduction of its crystallinity and Tm was evidenced. So, while the EG [this work] or AG [12] influenced the crystallinity (xPCL) of HOPCLOH, once the PEUs were prepared using HOPCLOH as a precursor, the xPCL was reduced.
In Table 4, the mechanical properties of PEUs derived from HOPCLOH [Mn(NMR) = 1240–1510 Da] are shown. The modulus from PEU4 to PEU16 oscillated from 6.1 to 2.1 MPa and with high elongation at break (1671–2432%), these results showed an elastomeric behavior. It is well known that the crystallinity can affect the mechanical properties of semicrystalline polymers enhances their strength. Usually, the crystallinity influences the modulus, so EG substituents affected the crystallinity of HOPCLOH as a precursor. In the case of PEUs, EG substituents affected the crystallinity of PCL segments and eventually the reduction of modulus values. The stress at break was relatively high from 32.7 to 28.5 MPa for the samples PEU4 to PEU10, respectively. However, when the EG was longer [−(CH2CH2O)m-, where m = 6 and 8] in the samples PEU12 and PEU16 the values of stress at break were reduced to 5.0 and 4.6 MPa, respectively. Additionally, PEU16 had the lowest value of modulus (2.1 MPa) and the highest value of strain at break (2432%). Hence, PEU12 and PEU16 in comparison with the rest of PEUs presented poor mechanical properties. So, the type of EG had influences on the mechanical properties of the derivatives of PEUs such as modulus and stress at break. On another hand, for PEUs derived from HOPCLOH with relative high Mn and crystallinity produced a proportional increase in the modulus. To visualize this effect a couple of samples of PEUs using HOPCL8aOH [Mn(NMR) = 2470] and HOPCL10aOH [Mn (NMR) = 2610] (two last lines in Tables 1 and 2) as precursors are shown in Fig. 8. Observing the graphic is evident that samples PEU8a and PEU10a exhibited a different profile respect to PEU8 and PEU10, this is attributed to the high content in the crystallinity of PCL in PEU8a and PEU10a (Table 4) with a clear yielding and high values of modulus (100–126 MPa) that involves a strong material, resistant to the deformation, and with an evident plastic behavior relative to PEU8 and PU10.
Finally, this work contributes to the knowledge in the area of the synthesis and characterization of degradable and biodegradable poly(ester-urethanes) (PEUs) that can be potentially used as biomaterials.
Conclusions
The effect of different substituents derived from ether group (EG) was analyzed in the α,ω-hydroxy telechelic poly(ε-caprolactone) (HOPCLOH), the EG was inserted in the main chain of HOPCLOH [HO-PCL-(CH2-CH2-O)m-PCL-OH, where m = 2, 3, 4, 5, 6, and 8] with a number average molecular weight (Mn) in the range of oligomers (Mn = 1240–1510 Da). The crystallinity of PCL decreased inversely proportional respect to the size of EG (from 48 to 38%) due to the disruption of the EG in the chains of HOPCLOH. Complementary, HOPCLOH and 1,6-hexamethylene diisocyanate (HDI) were precursors of the poly(ester-urethanes) (PEUs), the effect of EG on the mechanical properties of PEUs was detected, where an elastomeric behavior was observed, in this sense, PEUs species with long EG [m = 6 and 8] presented poor mechanic properties. On the other hand, when the Mn of HOPCLOH precursor was increased the PEUs showed higher values of modulus confirming that the crystallinity of PCL affected the mechanical properties and a plastic profile was evidenced. Finally, the use and selection of ether diols as initiators in the ROP of CL to synthesize HOPCLOH was not trivial because these EG substituents affected the crystallinity, and the mechanical properties of their PEUs.
References
Alger M (2017) Polymer science dictionary3rd edn. Dordrecht, Springer
Jakisch L, Garaleh M, Schäfer M, Mordvinkin A, Saalwächter K, Böhme F (2018). Macromol Chem Phys 219:1700327
Báez JE, Marcos-Fernández A, Galindo-Iranzo P (2011). Polym-Plast Technol Eng 50:839–850
Jeong K-H, Park D, Lee Y-C (2017). J Polym Res 24:112
Erdagi SI, Doganci E, Uyanik C, Yilmaz F (2016). React Funct Polym 99:49–58
Uyar Z, Öncel A (2018). J Polym Res 25:245
Lu Y, Cao J, Huang J, Xiong Z, Chen H, Xiong C, Chen D (2017). J Polym Res 24:200
Mandal M, Monkowius U, Chakraborty D (2016). J Polym Res 23:220
Báez JE, Marcos-Fernández A, Lebrón-Aguilar R, Martínez-Richa A (2006). Polymer 47:8420–8429
Sung S-J, Yun YH, Lee S, Park J-K, Kim D-H, Cho KY (2010). React Funct Polym 70:622–629
Guillaume SM (2013). Eur Polym J 49:768–779
Báez JE, Marcos-Fernández A, Martínez-Richa A, Galindo-Iranzo P (2017). Polym-Plast Technol Eng 56:889–898
Báez JE, Marcos-Fernández A, Galindo-Iranzo P (2011). J Polym Res 18:1137
Báez JE, Marcos-Fernández A, Navarro R, García C (2017). J Polym Res 24:199
Báez JE, Marcos-Fernández A (2011). Int J Polym Anal Charact 16:269–276
Takizawa K, Tang C, Hawker CJ (2008). J Am Chem Soc 130:1718–1726
Báez JE, Zhao R, Shea KJ (2017). Ind Eng Chem Res 56:10366–10383
Huang M-H, Li S, Coudane J, Vert M (2003). Macromol Chem Phys 204:1994–2001
Huang M-H, Li S, Vert M (2004). Polymer 45:8675–8681
Naguib HF, Abdel Aziz MS, Sherif SM, Saad GR (2011). J Polym Res 18:1217–1227
Báez JE, Marcos-Fernández A (2012). React Funct Polym 72:349–357
Báez JE, Ramírez D, Valentín JL, Marcos-Fernández A (2012). Macromolecules 45:6966–6980
Ping P, Wang W, Chen X, Jing X (2005). Biomacromolecules 6:587–592
Panwiriyarat W, Tanrattanakul V, Pilard J-F, Pasetto P, Khaokong C (2013). J Appl Polym Sci 130:453–462
Ma Z, Hong Y, Nelson DM, Pichamuthu JE, Leeson CE, Wagner WR (2011). Biomacromolecules 12:3265–3274
Lin C-Y, Hsu S-H (2015). J Biomed Mater Res B Appl Biomater 103B:878–887
Tatai L, Moore TG, Adhikari R, Malherbe F, Jayasekara R, Griffiths I, Gunatillake PA (2007). Biomaterials 28:5407–5417
Rattanapan S, Pasetto P, Pilard J-F, Tanrattanakul V (2016). J Polym Res 23:182
Wu C-L, Tsou C-Y, Tseng Y-C, Lee H-T, Suen M-C, Gu J-H, Tsuo C-H, Chiu S-H (2016). J Polym Res 23:263
Yuan J, Sang Z, Zhao J, Zhang Z, Zhang J, Cheng J (2017). J Polym Res 24:88
Li SQ, Zhao JB, Zhang ZY, Zhang JY, Yang WT (2015). Polymer 57:164–172
Li SQ, Sang ZH, Zhao JB, Zhang ZY, Cheng J, Zhang JY (2016). Eur Polym J 84:784–798
Báez JE, Martínez-Rosales M, Martínez-Richa A (2003). Polymer 44:6767–6772
Dey P, Hemmati-Sadeghi S, Haag R (2016). Polym Chem 7:375–383
Chausson M, Fluchère A-S, Landreau E, Aguni Y, Chevalier Y, Hamaide T, Adbul-Malak N, Bonnet I (2008). Int J Pharm 362:153–162
Báez JE, Ramírez-Hernández A, Marcos-Fernández A (2011). Int J Polym Anal Charact 16:377–389
Sigma-Aldrich is now Merck. Thermal transitions of homopolymers: glass transition & melting point. https://www.sigmaaldrich.com/technical-documents/articles/materials-science/polymer-science/thermal-transitions-of-homopolymers.html. Accessed 03 Dec 2018
Piao L, Dai Z, Deng M, Chen X, Jing X (2003). Polymer 44:2025–2031
Zamani S, Khoee S (2012). Polymer 53:5723–5736
Báez JE, Marcos-Fernández A, Navarro, R. Chem Pap accepted manuscript
Acknowledgments
José E. Báez thanks the “Consejo Nacional de Ciencia y Tecnología” (CONACYT) (Proyecto CONACYT Ciencia Básica 284893), Dirección de Apoyo a la Investigación y al Posgrado (DAIP) at University of Guanajuato (UG), and “Sistema Nacional de Investigadores (SNI)” in México for financial support of the work. José E. Báez also thanks to Ángel Marcos-Fernández for believing in these ideas and providing financial support for the reagents through the project MAT2017-87204-R from the Ministry of Economy and Competitiveness (MINECO) of Spain. José E. Báez also thanks to the UG for the recent opportunity to work as an Assistant Professor. Marvin was used for drawing, displaying, and characterizing chemical structures, substructures, and reactions (Marvin Sketch 6.1.3, 2013, ChemAxon; http://www.chemaxon.com); a free software program with an academic license was provided by ChemAxon. Finally, José E. Báez thanks to Gema Reina Mendieta for the acquisition of the NMR spectra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Báez, J.E., Marcos-Fernández, Á., Navarro, R. et al. A systematic study of macrodiols and poly(ester-urethanes) derived from α,ω-hydroxy telechelic poly(ε-caprolactone) (HOPCLOH) with different ether [CH2CH2O]m groups. Synthesis and characterization. J Polym Res 26, 32 (2019). https://doi.org/10.1007/s10965-018-1682-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-018-1682-4