Abstract
Ether group (E) diols [HO(CH2CH2O)xH, x = 2, 3, 4, 5, 6, and 8] and polyethylene glycol (PEG) [HO(CH2CH2O)xOH] were used as initiators in the ring-opening polymerization (ROP) of ε-caprolactone (CL) catalyzed by ammonium decamolybdate to synthesize the poly(ε-caprolactone) (PCL) macrodiols such as HOPCL–E–PCLOH and HOPCL–PEG–PCLOH, respectively. The effect of E or PEG on the crystallinity of the PCL segments (xPCL) in HOPCL–E–PCLOH homopolymer and HOPCL–PEG–PCLOH triblock copolymer was evidenced, respectively, where a weight percent of E or PEG was inversely proportional to the xPCL; this effect was explained due to that the ether segments are causing a partial disruption on the crystalline domain of PCL. A couple of species of poly(ester–ether–urethanes) (PEU) derived from HOPCL–E–PCLOH and HOPCL–PEG–PCLOH with 1,6-hexamethylene diisocyanate (HDI) were prepared, and these PEU showed an elastomeric behavior. Comparing two samples of PEU prepared from E (MW = 370 g/mol) (PEUE) and PEG (Mn = 408 g/mol) (PEUPEG), a parallel profile of their mechanical properties was seen. Eventually, HOPCL–E–PCLOH and HOPCL–PEG–PCLOH species showed similarities in terms of crystallinity and elastomeric behavior from their PEU derivatives. In addition, HOPCL–E–PCLOH and HOPCL–PEG–PCLOH and PEU samples were characterized by 1H NMR, FT-IR, GPC, DSC, and mechanical properties. Hence, homopolymers and triblock copolymers derived from PCL macrodiols with E or PEG segments, respectively, and their PEU had similarities in terms of chemical structure and physical properties
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poly(ε-caprolactone) (PCL) is a biodegradable aliphatic polyester that is preferentially synthesized by ring-opening polymerization (ROP) of ε-caprolactone (CL) (Scribanti et al. 2016 and Yao et al. 2014). The ingredients involved in the ROP of CL are a monomer (CL), initiator, and catalyst (Báez et al. 2003). An important characteristic during the synthesis of PCL is that the use of a solvent can be avoided (green chemistry) by bulk polymerization to high temperatures with respect to the regular melting point of PCL (> 60 °C) (Báez et al. 2011a, b; Erdagi et al. 2016). In the ROP of CL, the initiator provides the functionality of PCL; it means that alcohol [ROH] (Báez et al. 2011a, b; Erdagi et al. 2016), diol [HOROH] (Báez et al. 2006), triol [HOR(OH)2] (Meier et al. 2004), and tetraol [(HO)2R(OH)2] (Choi et al. 2005) can produce α-hydroxy PCL (Báez et al. 2011a, b; Erdagi et al. 2016), α,ω-hydroxy telechelic PCL (or PCL diol) (Báez et al. 2006), PCL triol (Meier et al. 2004; Brzeska et al. 2017), and PCL tetraol (Choi et al. 2005), respectively. PCL diol (HOPCLOH) represents an important functionality; HOPCLOH is the precursor of a family called poly(ester-urethanes) (PEU) that can be used potentially in a biomedical area (Lin and Hsu 2015; Ma et al. 2011). A route to synthesize the PCL diol is using an aliphatic or ether group (E) diol as an initiator, such as ethylene glycol [HOCH2CH2OH] (Ping et al. 2005) and diethylene glycol [HOCH2CH2OCH2CH2OH] (Lin and Hsu 2015), respectively. For example, Lin and Hsu (2015) used the PCL diol with an ether group in the main chain (HOPCL–E–PCLOH) as a precursor of biodegradable polyurethane microspheres. Ping et al. (2005) synthesized a series of segmented polyurethanes with PCL soft segment using PCL diol as a precursor, and a significant shape-memory effect was displayed.
Another species of interest is a triblock copolymer derived from PCL, especially, the α,ω-hydroxy telechelic PCL–polyethylene glycol–PCL (HOPCL–PEG–PCLOH) (Colwell et al. 2015; Bai et al. 2016) due to the intrinsic amphiphilic environment in the main chain, where PCL and PEG are hydrophobic and hydrophilic segments, respectively. Recently, calcium hydride (Colwell et al. 2015) and N-heterocyclic carbene (Bai et al. 2016) have been used as new catalysts to synthesize HOPCL–PEG–PCLOH. Guo et al. (2017) reported curcumin-loaded nanoparticles derived from HOPCL–PEG–PCLOH as a useful system for controlled drug release. Hence, HOPCL–PEG–PCLOH has been used in interdisciplinary studies with different areas of the sciences such as chemistry, biomaterials, microbiology, pharmacology, nanomaterials, and medicines; all of them were reported in the recent years (Pazarçeviren et al. 2017; Guo et al. 2017). HOPCL–PEG–PCLOH is synthesized by ROP of CL using polyethylene glycol (PEG) [HO(CH2CH2O)xH] as a macroinitiator (Bai et al. 2016; Colwell et al. 2015). HOPCL–PEG–PCLOH is also an important precursor of poly(ester–ether–urethanes) (PEU); PEU represents materials with interesting properties in a biomedical area (You et al. 2008).
The significance of this work is to understand the effect of E on the macrodiols properties derived from HOPCL–E–PCLOH and HOPCL–PEG–PCLOH [that usually had been synthesized and reported for different potentials applications (Hlaváč et al. 2018; Yin et al. 2015)] due to their similarities regarding ether functional groups and polyethylene glycol segment. Recently, in our research, we explored the differences and similarities between homopolymers and diblock copolymers (Báez et al. 2017a, b).
The topic of this work is a comparison between two different polymeric species with one factor in common, the ether segment: 1) ether group (E) diols as initiators HO(CH2CH2O)xH (where x = 2, 3, 4, 5, 6, and 8) in the synthesis of HOPCL–E–PCLOH homopolymer and 2) polyethylene glycol (PEG) as macroinitiators HO(CH2CH2O)xH in the preparation HOPCL–PEG–PCLOH triblock copolymer. In this sense, a simple question can illustrate our interest in this contribution: how is the effect of the E or PEG segment on the homopolymer (HOPCL–E–PCLOH) or triblock copolymer (HOPCL–PEG–PCLOH)? (Scheme 1) To our knowledge, a comparison between homopolymers and triblock copolymers derived from PCL and ether groups (or PEG) has not been reported. In addition, all polymeric species were characterized using different analytical techniques such as 1H NMR, FT-IR, GPC, and DSC.
Experimental
Materials
Diethylene glycol, triethylene glycol, tetraethylene glycol, pentaethylene glycol, hexaethylene glycol, octaethylene glycol, polyethylene glycol (PEG) (Mn = 200, 400, 600, 1000, 1500 y 2000 g/mol), and ε-caprolactone (CL) were purchased from Aldrich Chemical Co. Ammonium heptamolybdate tetrahydrate (NH4)6[Mo7O24].4H2O (Hep) (Fluka) was ground with a pestle and mortar before use.
A typical procedure for the synthesis of α,ω-hydroxy telechelic poly(ε-caprolactone) (HOPCL–E106–PCLOH or macrodiol) by ammonium decamolybdate as a catalyst and diethylene glycol (DEG) [HO(CH2CH2O)2H, M W = 106.12 g/mol] as the initiator
Polymerization was performed in absent of solvent (bulk polymerization) in a dried 25 ml round-bottom flask. Ammonium heptamolybdate tetrahydrate [(NH4)6[Mo7O24]·4H2O (Hep), 3 mg], ε-caprolactone (CL) (50 mmol), 5.707 g), and diethylene glycol (DEG) (5 mmol, 530 mg) were charged and heated to reflux by stirring them in an oil bath at 150 °C for 30 min (molar ratio CL/Hep = 20,600 and CL/DEG = 10). Ammonium decamolybdate (NH4)8[Mo10O34] was obtained in situ in the solid state by thermal decomposition of ammonium heptamolybdate [(NH4)6[Mo7O24] (Báez et al. 2003).
The product α,ω-hydroxy telechelic poly(ε-caprolactone) (HOPCL-E106-PCLOH, where E and 106 are indicating ether group and the molecular weight of the ether group diol used as the initiator, respectively) synthesized was analyzed without purification. Number-average molecular weight (Mn) and conversion were monitored by 1H NMR. After reaction time, an aliquot of crude of the reaction was dissolved in CDCl3 and derivatized with two drops of trifluoroacetic anhydride (TFAA) to prevent overlapping between methylene attached to hydroxyl and diethylene glycol groups and analyzed by 1H NMR (Báez et al. 2011a, b). In 1H NMR spectrum, the peaks at 2.36 [–CH2–CO–O–, Ipol, repetitive unit], 3.82 [F3C–CO–O–CH2–CH2–O–CH2–CH2–O–CO–, Ieg, monosubstitution of diethylene glycol], and 3.76 [–CO–O–CH2–CH2–O–CH2–CH2–O–CO–, Ieg, bisubstitution of diethylene glycol] were used to quantify the Mn in two steps:
(1) The degree of polymerization (DP). DP(NMR) = Ipol/#Hpol ÷ Ieg/#Heg. Ipol and Ieg represent the integrals of the methylenes obtained by 1H NMR from the polyester [–CH2–CO–O–] and diethylene glycol group [F3C–CO–O–CH2–CH2–O–CH2–CH2–O–CO– and –CO–O–CH2–CH2–O–CH2–CH2–O–CO–] peaks, respectively, #Hpol and #Heg represent the number of protons that contributed to the peaks. Finally, the equation is DP(NMR) = Ipol/2 ÷ Ieg/4.
(2) The number-average molecular weight (Mn). Mn(NMR) = (MW(CL)).(DP(NMR)) + MW(diol), where MW is the molecular weight of the repetitive unit (CL) and diol (diethylene glycol), respectively; DP(NMR) was previously calculated in step 1. Mn(calcd) = 1250, Mn(NMR) = 1300 (Conv. = 99%), Mn(GPC) = 2720, Mw/Mn = 1.32. IR (cm−1) 3446 (ν, OH, PCL), 2942 (νas, CH2, PCL), 2865 (νs, CH2, PCL), 1721 (ν, C=O, PCL), 1470 (δs, CH2, PCL), 1162 (νas, C–(C=O)–O, PCL), 1044 (νas, O–C–C, PCL), and 732 (ρ, CH2, PCL). NMR data for HOPCL4OH. 1H NMR after derivatization with TFAA (400 MHz, CDCl3, ppm): δ 4.50 [F3C–CO–O–CH2–CH2–O–, DEG monosubstitution and unreacted DEG (4)], 4.35 [–CO–CH2–CH2–CH2–CH2–CH2–O–CO–CF3, PCL (f)], 4.27 [–CO–O–CH2–CH2–O–CH2–CH2–O–CO–, DEG bisubstitution (2) and F3C–CO–O–CH2–CH2–O–CH2–CH2–O–CO–, DEG monosubstitution (2)], 4.10 [(–CO–CH2–CH2–CH2–CH2–CH2–O–)n, PCL (d)], 3.82 [F3C–CO–O–CH2–CH2–O–, DEG monosubstitution and unreacted DEG (3)], 3.76 [–CO–O–CH2–CH2–O–CH2–CH2–O–CO– DEG bisubstitution (1) and F3C–CO–O–CH2–CH2–O–CH2–CH2–O–CO–, DEG monosubstitution (1)], 2.35 [(–CO–CH2–CH2–CH2–CH2–CH2–O–)n, PCL (a)], 1.77 [–CO–CH2–CH2–CH2–CH2–CH2–O–CO–CF3, PCL (e)], 1.66 [(–CO–CH2–CH2–CH2–CH2–CH2–O–)n, PCL (b)], 1.38 [(–CO–CH2–CH2–CH2–CH2–CH2–O–)n, PCL (c)]. The triblock copolymer HOPCL–PEG–PCLOH samples were prepared analogously to that previously described but with different types Mn of PEG as macroinitiators.
A typical procedure for the synthesis of poly(ester–ether–urethane) (PEU) derived from HOPCL–PEG408–PCLOH and 1,6-hexamethylene diisocyanate (HDI)
The reaction was carried out in a 25-ml round-bottom flask previously dried. 2.23 g of HOPCL–PEG408–PCLOH [Mn(NMR) = 1570] was charged [according to 1H NMR analysis, it is assumed that 8% of unreacted diol (HOPEGOH) is present in the polymer sample, so this fraction was considered in the preparation, Mn = 1440 (1.54 mmol)], and then, 1,6-hexamethylene diisocyanate (HDI) (1.75 mmol, 294 mg) and tin(II) 2-ethylhexanoate [Sn(Oct)2] (34 mg, ~ 3 drops) were added as diisocyanate and catalyst, respectively, and dissolved in 8 ml of 1,2-dichloroethane (DCE). A molar ratio 1:1.14 (HOPCL–PEG408–PCLOH:HDI) with a slight excess of HDI was used to prevent the reaction of moisture present in the HOPCL–PEG408–PCLOH. In addition, a drying tube to prevent the moisture was adapted on the top of a reflux system. After 1 h of reaction at 80 °C, a fresh portion of solvent was added (~ 1 ml) to prevent a high viscosity. Then, the reaction mixture was stirred for another 2 h at 80 °C. The PEUPEG408 film was obtained by casting in a leveled Teflon surface within a fume cupboard. The cast solution (at 80 °C) was covered with a conical funnel to protect it from dust and to avoid an excessively fast solvent evaporation an allowed to stand a room temperature for 12 h. Next, the PEU film was released and dried under vacuum. Using the same methodology, other different PEUs were synthesized. Mn(GPC) = 122,530, Mw/Mn = 1.59. IR (cm−1) 3326 (ν, N–H, urethane), 2938 (νas, CH2, PCL), 2865 (νs, CH2, PCL), 1720 (ν, C=O, PCL), 1684 (ν, C=O, urethane), 1467 (δs, CH2, PCL), 1161 (νas, C–(C=O)–O, PCL), 1044 (νas, O–C–C, PCL), and 731 (ρ, CH2, PCL). In all samples of PEUs, the band at 2250 cm−1 detected by FT-IR and attributed to diisocyanate group in the HDI was not observed.
Polymer characterization
Nuclear magnetic resonance (NMR) 1H NMR was recorded at room temperature on a Varian Inova or Mercury 400 MHz (400 MHz 1H and 100 MHz 13C). CDCl3 was used as a solvent, and all spectra were referenced to the residual solvent CDCl3 [δ (ppm) 7.26 (1H)]. Fourier transform infrared spectroscopy (FT-IR) Homopolymers (HOPCLOHs) and poly(ester–ether–urethanes) (PEUs) films were recorded with attenuated total reflectance spectroscopy (ATR) accessory in a Perkin–Elmer Spectrum One FT-IR spectrometer. Differential scanning calorimetry (DSC) Thermograms were performed in a Mettler Toledo DSC822e instrument. Three scans were obtained with two heating (25–100 °C and − 100 to 100 °C) and one cooling (100 to − 100 °C) between them, at a rate of 10 °C/min and under a nitrogen purge. The degree of crystallinity (xPCL) for PCL was calculated from the endothermic peak area (ΔHm) by xPCL = ΔHm/ΔH 0m , where H 0m is the heat of fusion for perfect PCL (135.3 J/g) (Crescenzi et al. 1972) crystals; in the case for PEG H 0m , the heat of fusion for perfect PEG was 188.1 J/g (Li et al. 2013; Martuscelli et al. 1985). Complementary, For PEG macrodiol samples, a TA Instruments Q2000 was used. Three scans (25–100 °C, 100 to − 40 °C, and − 40 °C to 100 °C) were performed using a heating rate of 10 °C/min and cooling the instrument between runs under a nitrogen purge. Gel permeation chromatography (GPC) HOPCL–E–PCLOH and HOPCL–PEG–PCLOH: GPC measurements were determined using a Waters gel permeation chromatograph equipped with a Waters 1515 isocratic high-performance liquid chromatography (HPLC) pump and Waters 2414 refractive index (RI) detector. A set of three Waters columns conditioned at 35 °C were used to elute samples at the flow rate of 1 mL/min HPLC grade tetrahydrofuran (THF). Polystyrene standards (Polymer Laboratories) were used for calibration. Mechanical properties The mechanical properties were measured in an MTS testing machine equipped with a 100 N load cell. Type 3 dumbbell test pieces (according to ISO 37) were cut from the films. A crosshead speed of 200 mm/min was used. The strain was measured from crosshead separation and referred to 12 mm initial length. At least three samples were evaluated for each PEU.
Results and discussion
α,ω-Hydroxy telechelic poly(ε-caprolactone) using ether group diols (HOPCL–E–PCLOH) and polyethylene glycol (PEG) (HOPCL–PEG–PCLOH) as initiators and macroinitiators
A family of six different molecules derived from ether group (E) diols such as ethylene glycol [HO(–CH2–CH2–O–)xH, where x = 2, 3, 4, 5, 6, and 8] (HOEOH) were used as initiators in the ring-opening polymerization (ROP) of ε-caprolactone (CL) in the presence of decamolybdate anion as catalyst under bulk polymerization at 150 °C for 30 min (Scheme 1). After the reaction time, a high conversion (99%) was obtained (Table 1). Therefore, the values of the degree of polymerization (DP) calculated by end-group analysis (1H NMR) [DP(NMR)] were similar to DP(calcd) (obtained by monomer and initiator feed); therefore, this is evidence of control of polymerization. In the same way, the number of average molecular weight (Mn) Mn(NMR) is close to Mn(calcd). Thus, all the initiators acted as transfer agent during the polymerization. It is well known that in the ROP of CL different types of alcohol or diol are transfer agents in the presence of a metallic catalyst (Báez et al. 2006 and Erdagi et al. 2016). To have a better perspective of the effect of E substituents (with low MW) on the α,ω-hydroxy telechelic poly(ε-caprolactone) (HOPCL–E106–370–PCLOH, Ex where x is the molecular weight of the E diol), a low value of DPPCL ~ 10 was synthesized, where DP(calcd) = CL/initiator. From HOPCL–E106–PCLOH to HOPCL–E370–PCLOH the weight percent (wt%) of the E inserted in the PCL main chain was from 8 to 24%, these percentages are important due to involving a factor to compare with polyethylene glycol (PEG) segment in triblock copolymers (HOPCL–PEG–PCLOH) (see Table 1). In the penultimate column of Table 1, the values of Mn(GPC) are visualized; it is evident that the Mn(GPC) is higher than Mn(calcd) or Mn(NMR); approximately, with the double value of the Mn(calcd), which is attributed to the polystyrene standards used in the calibration curve; in addition, the polydispersity of the HOPCL–E–PCLOH (Mw/Mn = 1.27–1.47) was moderate.
HOPCL–PEG–PCLOH was prepared according to the same method of HOPCL–E–PCLOH but using polyethylene glycol (HOPEGOH) as a macroinitiator. In Table 1, six different samples of oligomeric species from a triblock copolymer of HOPCL–PEG–PCLOH with a variation of PEG (16–69 wt%) segment are seen. Unimodal distribution with some moderate values of polydispersity and a good approach between Mn(calcd) and Mn(NMR) were observed, such as in the previous HOPCL–E–PCLOH species. Practically, homopolymers and triblock copolymers have similar DP(NMR) ~ 10 of PCL, but a significant variation of Mn(NMR) due to the contribution of E relative to PEG segments.
The chemical nature of homopolymers (HOPCL-E–PCLOH) and triblock copolymers (HOPCL–PEG–PCLOH) was analyzed by nuclear magnetic resonance (NMR). In Fig. 1, 1H-NMR spectra of three distinct species of HOPCL–E–PCLOH with a significant increase in the number of methylenes [(a) 10, (b) 12, and (c) 16] in the E are showed. Due to that methylene attached to the hydroxyl group which is regularly overlapping with methylenes bonding to oxygen in the E in a regular 1H-NMR, the derivatization reaction using trifluoroacetic anhydride [CF3(O=C)–O–(C=O)CF3, TFAA] was realized (Báez et al. 2006, 2011b). In the derivatization reaction (Fig. 1), the hydroxyl terminal groups [–CH2–OH, δ 3.64] react with the TFAA to produce trifluoroacetate ester groups [f, –CH2–O–(C=O)CF3, δ 4.35]. Complementary signals for PCL [d, –CH2–O–(C=O)–, δ 4.10 and a, –O–(C=O)–CH2–, δ 2.35], E [5, –CH2–O–, δ 3.70], bisubstitution E [1, –CH2–CH2–O–, δ 3.76 and 2, –CH2–CH2–O–, δ 4.25] and monosubstitution E [3, –CH2–CH2–O–(C=O)CF3, δ 3.82 and 4, –CH2–CH2–O–(C=O)CF3, δ 4.50] were observed. In addition, in the previous contributions for macrodiols, one fraction of unreacted diol had been quantitative in the PCL diols (Báez et al. 2017a), and the peaks of unreacted E diol feed are overlapping with signals of monosubstitution E species. The peak number 5 attributed to E is increasing proportionally from Fig. 1a–c. On the other hand, 1H-NMR spectra of triblock copolymers derived from HOPCL–PEG–PCLOH are similar to the HOPCL–E–PCLOH (Fig. 2). Therefore, the differentiation between the homopolymer or triblock copolymer by NMR spectra is not obvious. Thus, 1H NMR is a good technique to visualize similarities between homopolymers and triblock copolymers.
In Table 2, thermal properties for HOPEGOH macrodiols are seen. Samples from HOPEG408OH to HOPEG2260OH were semicrystalline oligomers with a melting temperature (Tm) and crystallinity (xPEG) proportional to the Mn(NMR) (Fig. 3). However, for HOPEG240OH, the melting point was not observed during all the experiments (from − 40 to 100 °C), so HOPEG240OH is an amorphous oligomer.
DSC thermograms (second heating) of a HOPEG408OH and b HOPEG608OH used as macroinitiators (see Table 2)
The results of the thermal properties investigation of HOPCL–E–PCLOH and HOPCL–PEG–PCLOH are in Table 3. In all the samples, a perceptible glass transition temperature (Tg) was observed, where analogous values (from − 70 to − 65 °C) are evident for the two species, indicating an amorphous domain in the semicrystalline PCL segment of the homopolymer or triblock copolymer. The melting point (Tm) exhibited two endothermic peaks in all the species (Fig. 4), this effect is attributed to two distinct environments of crystallites from PCL segments, where the crystallites with low Tm are embedded in amorphous domains, and the crystallites with relative high Tm are immersed in zones more crystallines.
When two samples with similar molecular weight of E diol (monodisperse initiator, MW = 370 g/mol) (Fig. 4, down) and PEG (polydisperse macroinitiator, Mn = 408 g/mol) (Fig. 4, top) were used as initiators and inserted in the main chain of PCL, different melting temperatures were visualized for HOPCL–PEG–PCLOH with two characteristics endothermic zones, the first zone at − 13 °C is not corresponding to PEG, on the contrary, this peak was attributed to an impurity, probably ethylene glycol (Tm = − 13 °C); the second zone at 34 and 41 °C was assigned to PCL segment. In the case of HOPCL–E–PCLOH, only the Tm of PCL segment (31 and 39 °C) was visualized. This effect is explained, because the PEG (400 g/mol) and E (370 g/mol) segments have low molecular weight and their precursors (diol and macrodiol) are liquids a room temperature (25 °C) and amorphous; so, around 30 °C, there is not fusion of E or PEG segments in the homopolymer or triblock copolymer, respectively, only from PCL segments.
The effect of E on the HOPCL–E–PCLOH has a decrease in the crystallinity values of PCL segment from 48 (diethylene glycol) to 38% (octaethylene glycol) with a DP (~ 10) similar of PCL (Fig. 5a). An analogous effect for HOPCL–PEG–PCLOH was visualized but with an accentuated decrease, because the PEG segments have a long-chain and molecular–weight distribution respect to E (Fig. 5b). Therefore, a long ether substituent (E or PEG) causes a disruption in the PCL crystalline domains favoring the amorphous domains. Complementary, when the PEG segment has similar or relative high Mn respect to PCL segment in the HOPCL–PEG–PCLOH, an overlapping of melting points from both segments was observed; this effect is because the weight percent of PEG is gradually increasing and its enthalpy of fusion. Therefore, the enthalpy of fusion of PCL segment decreases in a proportional manner and its crystallinity.
The evidence regarding chemical nature (NMR and GPC) and thermals properties (DSC) show that oligomers derived from HOPCL–E–PCLOH or HOPCL–PEG–OH had a similar pattern of NMR spectra and DSC thermograms, and thus, the frontier between homopolymers and triblock copolymers have points in common.
Poly(ester–ether–urethane)
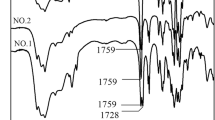
Poly(ester–ether–urethanes) (PEU) were synthesized from a homopolymer (HOPCL–E370–PCLOH, Mn(NMR) = 1510) and a triblock copolymer (HOPCL–PEG408–PCLOH, Mn(NMR) = 1570) and 1,6-hexamethylene diisocyanate (HDI), both macrodiols were selected due to their similar Mn and ether segment. Typically, HOPCL–E370–PCLOH and HDI were reacted with a molar ratio 1:1.14, respectively, using tin(II) 2-ethylhexanoate [Sn(Oct)2] and 1,2-dichloroethane (DCE) as a catalyst and solvent, respectively, at 80 °C for 3 h. In the case of PEUE370 derived from HOPCL–E370–PCLOH, to corroborate the functional groups, the formation of the urethane group [1685 cm−1 (C=O) and 1533 cm−1 (N–H)] and the ester group [1722 cm−1 (C=O)] was observed, the same pattern in the PEUPEG408 derived from HOPCL–PEG408–PCLOH was seen. Complimentary, the carbonyl group in the HDI [2250 cm−1 (C=O)] was not visualized.
In Table 4, the thermal properties of PEU are illustrated. Usually, the pattern of PEU samples showed two different transitions, Tg and Tm (Fig. 6). The Tg (− 55 °C) of PEU have an increase in the values with respect to the macrodiols (from − 70 to − 65 °C) due to the hydrogen bonding of urethanes groups. Tm (26–28 °C) and the crystallinity (xPCL) (18–19%) of PEU were decreasing in comparison with their macrodiols precursors, because the physical crosslinking from the urethane group restricts the possibilities to form crystalline domains of PCL.
In Fig. 7, mechanical properties of PEU derived from a homopolymer (PEUE370, E = 370 g/mol) vs triblock copolymer (PEUPEG408, PEG = 408 g/mol) with analogous molecular of E or PEG (Table 5) were analyzed, where the curves of mechanical properties showed an overlapping, exposing an elastomeric behavior for both samples. Therefore, the molecular weight distribution in PEG (PEUPEG408) did not have appreciable differences with respect to E (PEUE370) in terms of the stress–strain curve. However, the modulus exhibited a reduction that is consistent with the pattern in the penultimate column in Table 5. Therefore, the inclusion of EG or PEG in the PEU induces an elastomeric behavior where the modulus is more visible affected with PEG due to its molecular weight distribution.
Conclusions
Oligomers of the poly(ε-caprolactone) (PCL) macrodiols derived from homopolymers (HOPCL–E–PCLOH), and triblock copolymers (HOPCL–PEG–PCLOH) with two distinct types of substitution such as ether group (E) and polyethylene glycol (PEG) were synthesized to understand the effect of the ether substituents on their physical properties. Similarities between HOPCL–E–PCLOH and HOPCL–PEG–PCLOH in the NMR spectra and thermal properties (DSC) were found. For HOPCL–E–PCLOH and HOPCL–PEG–PCLOH, the crystallinity of PCL segment (DPPCL = 10) decreases proportionally according to the weight percent of E and PEG, respectively. The effect of E or PEG on the PCL is due to the ether segments that are causing a partial disruption of the crystalline domain of PCL. Both types of macrodiols were used to synthesize poly(ester-ether-urethanes) (PEU) derived from 1,6-hexamethylene diisocyanate (HDI), where the mechanical properties of the films of PEU have a similar elastomeric behavior for the two species, with low values of the modulus.
References
Báez JE, Martínez-Rosales M, Martínez-Richa A (2003) Ring-opening polymerization of lactones catalyzed by decamolybdate anion. Polymer 44:6767–6772
Báez JE, Marcos-Fernández A, Lebrón-Aguilar R, Martínez-Richa A (2006) A novel route to α,ω-telechelic poly(ε-caprolactone) diols, precursors of biodegradable polyurethanes, using catalysis by decamolybdate anion. Polymer 47:8420–8429
Báez JE, Marcos-Fernández A, Galindo-Iranzo P (2011a) On the effect of alkyl end group in poly(ε-caprolactone) oligomers: preparation and characterization. Polym Plast Technol Eng 50:839–850
Báez JE, Ramírez-Hernández A, Marcos-Fernández A (2011b) The effect of trifluoroacetic anhydride on poly(ε-caprolactone) (PCL) oligomers. Int J Polym Anal Charact 16:377–389
Báez JE, Marcos-Fernández A, Martínez-Richa A, Galindo-Iranzo P (2017a) Poly(ε-caprolactone) diols (HOPCLOH) and their poly(ester-urethanes) (PEUs): the effect of linear aliphatic diols [HO–(CH2)m–OH] as initiators. Polym Plast Technol Eng 56:889–898
Báez JE, Zhao R, Shea KJ (2017b) Synthesis of poly(methylene-b-ε-caprolactone) and poly(ε-caprolactone) with linear alkyl end groups: synthesis, characterization, phase behavior, and compatibilization efficacy. Ind Eng Chem Res 56:10366–10383
Bai J, Wu N, Wang Y, Li Q, Wang X, Zhang L (2016) Triblock and pentablock copolymerizations of ε-caprolactone with l-lactide catalyzed by N-heterocyclic carbene. RSC Adv 6:108045–108050
Brzeska J, Morawska M, Sikorska W, Tercjak A, Kowalczuk M, Rutkowska M (2017) Degradability of cross-linked polyurethanes based on synthetic polyhydroxybutyrate and modified with polylactide. Chem Pap 71:2243–2251
Choi J, Kim IK, Kwak SY (2005) Synthesis and characterization of a series of star-branched poly(ε-caprolactone)s with the variation in arm numbers and lengths. Polymer 46:9725–9735
Colwell JM, Wentrup-Byrne E, George GA, Schué F (2015) A pragmatic calcium-based initiator for the synthesis of polycaprolactone copolymers. Polym Int 64:654–660
Crescenzi V, Manzini G, Calzolari G, Borri C (1972) Thermodynamics of fusion of poly-β-propiolactone and poly-ε-caprolactone. Comparative analysis of the melting of aliphatic polylactone and polyester chains. Eur Polym J 8:449–463
Erdagi SI, Doganci E, Uyanik C, Yilmaz F (2016) Heterobifunctional poly(ε-caprolactone): synthesis of α-cholesterol-ω-pyrene PCL via combination of ring-opening polymerization and “click” chemistry. React Funct Polym 99:49–58
Guo F, Guo D, Zhang W, Yan Q, Yang Y, Hong W, Yang G (2017) Preparation of curcumin-loaded PCL–PEG–PCL triblock copolymeric nanoparticles by a microchannel technology. Eur J Pharma Sci 99:327–328
Hlaváč D, Klushina D, Tokarský J (2018) Interaction of antitumoral drug erlotinid with biodegradable triblock copolymers: a molecular modeling study. Chem Pap 72:2023–2034
Li Y, Ma Q, Huang C, Liu G (2013) Crystallization of poly(ethylene glycol) in poly(methyl methacrylate) networks. Mater Sci (Medžiagotyra) 19(2):1320–1392
Lin C-Y, Hsu S-H (2015) Fabrication of biodegradable polyurethane microspheres by a facile and green process. J Biomed Mater Res Part B Appl Biomater 103B:878–887
Ma Z, Hong Y, Nelson DM, Pichamuthu JE, Leeson CE, Wagner WR (2011) Biodegradable polyurethane ureas with variable polyester or polycarbonate soft segments: effects of crystallinity, molecular weight, and composition on mechanical properties. Biomacromol 12:3265–3274
Martuscelli E, Silvestre C, Glsmondi C (1985) Morphology, crystallization and thermal behavior of Poly(ethylene oxide)/poly(vinyl acetate) blends. Die Makromolekulare Chemie 186(10):2161–2176
Meier MM, Kanis LA, de Lima JC, Pires ATN, Soldi V (2004) Poly(caprolactone triol) as plasticizer agent for cellulose acetate films: influence of the preparation procedure and plasticizer content on the physico-chemical properties. Polym Adv Technol 15:593–600
Pazarçeviren E, Erdemli Ö, Keskin D, Tezcaner A (2017) Clinoptilolite/PCL–PEG–PCL composite scaffolds for bone tissue engineering applications. J Biomater Appl 31(8):1148–1168
Ping P, Wang W, Chen X, Jing X (2005) Poly(ε-caprolactone) polyurethanes and its shape-memory property. Biomacromol 6:587–592
Scribanti A, Bortoluzzi M, Gatto M (2016) New heteroscorpionate lanthanide complexes for ring-opening polymerization of ε-caprolactone and rac-lactide. Chem Pap 70:53–60
Yao L-H, Shao S-X, Jiang L, Tang N, Wu J-C (2014) Ring-opening polymerization of ε-caprolactone catalyzed by Brønsted acids. Chem Pap 68:1381–1389
Yin G, Zhao D, Wang X, Ren Y, Zhang L, Wu X, Nie S, Li Q (2015) Bio-compatible poly(ester-urethane)s based on PEG–PCL–PLLA copolymer with tunable crystallization and biodegradation properties. RSC Adv 5:79070–79080
You JH, Choi S-W, Kim J-H, Kwak Y-T (2008) Synthesis and microphase separation of biodegradable poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) multiblock copolymer films. Macromol Res 16:609–613
Acknowledgements
José E. Báez would like to thank the “Consejo Nacional de Ciencia y Tecnología (CONACYT), Proyecto 284893”, “Dirección de Apoyo a la Investigación y al Posgrado (DAIP) at University of Guanajuato (UG), Proyecto CIIC 103/2018” and “Sistema Nacional de Investigadores (SNI)” in México for the financial support of the work. José E. Báez would also like to thank Ángel Marcos Fernández for believing in these ideas and providing financial support for the reagents through the project MAT2017-87204-R from the Ministry of Economy and Competitiveness (MINECO) of Spain. José E. Báez would like to thank Gema Reina Mendieta and Dr. Rebeca Yasmín Pérez Rodriguez for the acquisition of the NMR spectra and DSC instrument, respectively. Finally, José E. Báez would also like to thank Irene Caso and Sonoko Kawawada for her support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Báez, J.E., Marcos-Fernández, Á. & Navarro, R. Similarities between homopolymers and triblock copolymers derived from poly(ε-caprolactone) (PCL) macrodiols (HOPCL–E–PCLOH and HOPCL–PEG–PCLOH) and their poly(ester-ether-urethanes): synthesis and characterization. Chem. Pap. 73, 1287–1299 (2019). https://doi.org/10.1007/s11696-019-00683-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-019-00683-3