Abstract
Atom transfer radical polymerization (ATRP) of styrene (St) initiated with trichloromethyl-terminated poly(vinyl acetate) was performed in the presence of CuCl/PMDETA as a catalyst system at 60 °C. PVAc-CCl3 telomers prepared from radical telomerization (without catalyst) and controlled radical telomerization (in the presence of Co(acac)2/DMF), were used in atom transfer radical polymerization (ATRP) to synthesize polystyrene-b-poly(vinyl acetate) (PSt-b-PVAc) diblock copolymers. The PVAc based block copolymers revealed a significant effect of Co(acac)2/DMF on PVAc macroinitiator which was used in the synthesis of highly ordered diblock copolymer on a well-defined microstructure. Diblock copolymers were characterized by 1H-NMR, DSC and GPC techniques. Reactivity ratios of St and VAc were calculated by the Mayo-Lewis (rSt = 0.563, rVAc = 0.081), Kelen-Todous (rSt = 0.583, rVAc = 0.074) and Finemann-Rose(rSt = 0.569, rVAc = 0.090) methods, which are in a good agreement with those reported for the conventional free-radical copolymerization of St and VAc. The number average molecular weights calculated from 1HNMR spectra were in good agreement with theoretically calculated values for block copolymers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Free radical polymerization is of great commercial and technological interest because of its mild reaction conditions, compatibility with a wide range of monomers, and high tolerance to impurities, water, functional groups and additives. Recently, the development of block copolymers with a well-defined polymer and copolymer were made possible by the discovery of several controlled/living radical polymerization systems that are based on rapid and reversible exchange between a low concentration of growing radicals and various types of dormant species [1, 2]. Controlled radical polymerization (CRP) is considered as one of the most spectacular advances in polymer chemistry in recent years because it can employ various strategies employed to controlled radical polymerization of vinyl acetate, atom-transfer radical polymerization (ATRP) has attracted the greatest attention since 1995[1–4]. PVAc-based block copolymers have been prepared by a combination of radical telomerization and ATRP.
However, the synthesis of well-defined PVAc block copolymers has been challenging because the controlled/living radical polymerization (CRP) of vinyl acetate (VAc) was difficult to control [5–8]. PVAc telomers were first synthesized by the telomerization of VAc with carbon tetrachloride, followed by ATRP of styrene (St) with the VAc telomers as macroinitiators [7, 8]. However, the low molecular weight (MW) polymer with a broad MW distribution of the VAc telomers makes it difficult to produce well-defined, high MW PVAc block copolymers through this method [3]. We have addressed earlier the synthesis of PVAc-based block copolymers containing styrene (St), methy(met)acrylate (MMA), PDMS via ATRP with CuCl/PMDETA catalyst system [3, 4, 7]. Telomerization is known as a very convenient approach for the preparation of macroinitiator having controlled molecular weights and required predetermined functionalities. By choosing an appropriate telogen, terminal functionalities are able to undergo the necessary chemical modifications. The most straightforward technique to obtain such an end group is to telomerize vinyl acetate monomer with CCl4 [1–3, 7]. In the past, vinyl acetate telomers were prepared in the controlled radical and radical telomerization by the same authors [7]. They were subsequently used as monomers for the synthesis of high molecular weight block copolymers. When chloroform is added as a transfer agent, a favorite hydrogen atom transfer will be observed for the radical initiator-induced telomerization that leads to much interesting monofunctional trichloromethylated telomers [7–10]. These telomers can be used as a new macromonomers in the synthesis of block copolymers with new properties [3, 7, 9–11]. 1HNMR study on the microstructure of VAc telomers from radical and controlled radical telomerization was reported earlier. It was also shown that the sequential arrangement of CH2 and CH groups of telomer was highly dependent on Co(acac)2 catalyst [7]. In this respect Co(acac)2 catalyst was able to provide a new syndiotactic route that produce telomers having controlled chain structures (PDI = 1.6) in the synthesis of macroinitiators used in ATRP [7, 10]. Reaction of the VAc telomers through radical telomerization and controlled radical telomerization with St indicated 62 % and 74 % yields of the diblock copolymer with dispersity of 1.8 and 1.3, respectively. Theoretical composition drifts in the comonomer mixture and copolymer chains were also investigated by calculation of monomer reactivity ratios. Results were compared with some experimental data to evaluate the accuracy of calculated reactivity ratios of St and PVAc.
In this study, thermal properties of the diblock copolymer was investigated by DSC which revealed a nearly available way to synthesize diblock polymers with styrene via ATRP initiated with trichloromethyl-terminated poly(vinyl acetate) telomer segments. Current experimental work aims to explain the effect of Co(acac)2/DMF catalyst and ligand on the synthesis of well-defined PVAc-based coolymers with a low PDI. This paper will also describe the synthesis of diblock copolymers with a well-defined PVAc microstructure and its potential use in membrane developments.
Experimental section
Material
Vinyl acetate (VAc) (Merck, >99 %) and styrene (St) (Merck, >99 %) were distilled over calcium hydride under reduced pressure before use. 2,2/-Azobisisobutyronitrile (AIBN, Fluka,≥98 %), choloroform-d (CDCl3, ARMAR Chemicals,Switzerland, 99.95 %), methanol (Merck,> 99 %), anhydrous tetrahydrofuran (THF) (Sigma-Aldrich, =99.9 %, inhibitor-free), cobalt acetylacetonate (Co(acac)2) (Merck, >99 %) and N,N,N′,N″,N″-pentamethyldiethylenetriamine (PMDETA) (Merck, 99.8 %) was also used as received were used as received. CuCl (Merck, 97 %) was washed by glacial acetic acid (three times), absolute ethanol and diethyl ether in turn and then dried under vacuum condition.
Synthesis of PVAc telomers from radical and controlled radical telomerization
A three-neck round-bottom flask equipped with a condenser and a magnetic stirrer was charged with VAc/CHCl3/AIBN/DMF/Co(acac)2 in concentration ratios of (1/1/0.01/0.01/0.05 and 1/1/0.01/0.01/0.0). The flask was immersed at 60 °C oil bath to initiate the reaction. The reaction was terminated after 5 h by removing the flask from the bath with unconverted monomer and chloroform was evaporated at room temperature. THF was then added to the product and refluxed for 2 h in order to decompose traces of the residual unreacted initiator. Finally, THF was evaporated and the polymer was dried under vacuum at 50 °C to reach constant weight (Scheme 1) [7].
ATRP of St and PVAc macroinitiator
The PVAc-based diblock copolymers were synthesized by copper (I)-mediated ATRP of styrene initiated with trichloromethyl-terminated poly(vinyl acetate) telomers from radical telomerization and controlled radical telomerization(Scheme 2) [3, 4, 7]. A required amount of CuCl (0.70 mmol, 0.069 g) was introduced into the three-neck round-bottomed flask equipped with a magnetic stirrer. The flask was sealed with a rubber septum and was cycled between vacuum and nitrogen three times. Reaction mixtures containing the required amounts of monomer, PVAc-CCl3 macroinitiator and PMDETA ligand were degassed by purging nitrogen for 30 min and then added to the flask. The molar ratio of reaction ingredients [Macroinitiator]0/[PMDETA]0/[CuCl]0/[Monomer]0 was kept constant for all experiments (1/4/2/200). The “freeze-pump-thaw” cycle was carried out three times to remove oxygen from the flask containing reaction mixture. Flask was sealed under vacuum and then immersed in a preheated oil bath at a desired temperature (i.e., 60 °C). The dried copolymer was redissolved in THF and passed through a short column of neutral alumina to remove the remaining copper catalyst [7, 10]. The samples were then dried under vacuum at 50 °C up to a constant weight and characterized by FTIR, 1HNMR, DSC, and GPC. The conversion was determined gravimetrically.
Characterization
PVAc-based block copolymers were dissolved in CDCl3 and characterized by 400 MHz 1HNMR spectroscopy (DRX 400 Bruker Avance) at ambient temperature. Apparent molecular weight and dispersity of the terpolymers dissolved in THF was determined by a Waters 150C gel permeation chromatography (GPC) equipped with a 104, 103, and 500 Ao set of Ultrastyragel columns and a refractive index (RI) detector. Polystyrene standards with the narrow molecular weight distributions and molecular weights in the range of analyzed molecular weights were used to calibrate the columns. Differential scanning calorimetry (DSC) measurements were performed by a Netzsch DSC 200 apparatus of heating rate of 10 °C/min [7, 8, 10].
Result and discussion
Synthesis of PVAc telomers
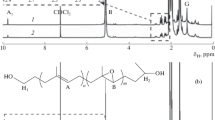
Figure 1 illustrates the 1HNMR spectra of PVAc telomers obtained from radical telomerization and controlled radical polymerization in the presence of Co(acac)2/DMF. Peaks of methylene protons in the vicinity of CCl3 (a) appear at 2.7–2.8 ppm. The methine protons of the first VAc unit (b) generated a small peak around 3.9 ppm. The peak at about 4.6–4.8 ppm was characteristic of the methine proton from repeating VAc units in backbone of the polymer (d) as well as the telomer molecules which show a greater intensity in the radical telomerization than the controlled telomerization, telomers with CCl3 α-end group’s increases in radical telomerization [7–11]. GPC traces during the ATRP reaction of PVAc-CCl3 telomers are shown in Fig. 2. Data from 1HNMR and GPC for the controlled radical telomerization and radical telomerization for 6 h at 60 °C are summarized in Table 1. Hydrogen atoms of methyl group in acetate appear at 2 ppm (g). Protons of the methylene for the repeating units (c) and the end chains (e) make a broad signals within the range of 1.6 to near 1.8 ppm [7]. The peak of AIBN at 7.0 ppm is small and negligible for the radical telomerization but is more significant for the controlled free radical telomerization, where the active site is Co(acac)2/DMF complex.
ATRP of St initiated with PVAc-CCl3
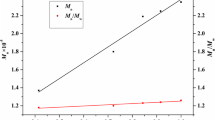
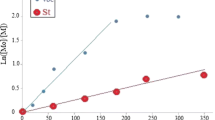
First-order kinetic polymerization plots of St initiated by PVAc-CCl3 macroinitiator and number average molecular weight, Mn, versus conversion in this polymerization are depicted in Fig. 3. The linear fit for telomerization in the presence of Co(acac)2/DMF and without catalyst demonstrated that the constant concentration of propagating radical species and radical termination reactions are not significant. This was further supported by the linear increase in the molecular weight upon conversion, indicating that the number of chains remains constant during the reaction. The molecular weight distribution is also decreased with progress of the polymerization, implying that nearly all the chains have started to grow simultaneously (Fig. 3). The controlled polymerization uncovered a narrow molecular weight distribution which was in a good agreement with both theoretical and experimental molecular weights (Table 2). GPC traces during the ATRP reaction of St with PVAc-CCl3 macroinitiator are shown in Fig. 4 (Table 2). The growing peak of diblock copolymer remained monomodal throughout the reaction, indicating that all the unreacted initiators have been removed prior to polymerization [5, 10, 11]. 1HNMR spectra of the PSt-b-PVAc diblock copolymers have been illustrated in Fig. 5. All signals of the 1HNMR spectra were assigned to their corresponding monomers and it can be declared that the synthesis of diblock copolymers have been proceeded successfully.
The 1HNMR of diblock showed a signal at 2.1–2.3 ppm corresponds to CH3 group of the PVAc segment, additional broad signals at about 6.6–7.1 ppm and 1.2–2.3 ppm were observed, which were assigned to the phenyl ring protons and aliphatic protons, respectively, of the PSt segment [4, 10]. The area ratio of 5H signal from PSt to that of vinyl acetate showed that the ratio of sequence lengths of PSt with respect to PVAc was found as 2.18 and 2.31 for telomerization without catalyst and controlled radical telomerization respectively which is related to higher reactivity ratio of St than VAc unit. It was possible to synthesize the PVAc-based diblock copolymers via ATRP of styrene in the presence of PVAc-CCl3 [3, 7, 10]. For styrene, the chemical shift effect of the phenyl is downfield, when the styrene content is higher (PVAc-CCl3 from controlled radical polymerization), the downfield effect is higher, so CH group of the styrene shifted to downfield region (Fig. 5b).
Block copolymers initiated with PVAc-CCl3 telomers from radical telomerization in the presence of Co(acac)2/DMF demonstrated syndiotactic microstructures (Pr = 0.69), while the block copolymers from PVAc-CCl3 telomers from radical telomerization were atactic (Pr = 0.48) [7]. To obtain more reliable monomer reactivity ratios, the cumulative average copolymer composition at moderate to high conversion was determined by 1H-NMR spectroscopy. Reactivity ratios of St and PVAc were calculated by the Mayo-Lewis (rSt = 0.563, rVAc = 0.081), Kelen-Todous (rSt = 0.583, rVAc = 0.074) and Finemann-Rose (rSt = 0.569, rVAc = 0.090), which are in a good agreement with those reported for the conventional free-radical copolymerization of St and VAc (Table 3). Good agreement between the theoretical and experimental composition drifts in the comonomer mixture and copolymer as a function of the overall monomer conversion were observed, indicating that the reactivity ratios calculated by copolymer composition at the moderate to high conversion are accurate. DSC thermograms of PSt-b-PVAc initiated with PVAc-CCl3 from controlled telomerization and radical telomerization at the range of −50 °C to 150 °C are depicted in Fig. 6. PSt-b-PVAc block copolymers revealed two glass transition temperatures in the range of 39.5–52.5 and 89–98 °C corresponding to PVAc and PSt segments respectively (Fig. 6a and b).
Conclusion
This paper has reported a new results from synthesis of well-defined poly(vinyl acetate) containing block copolymers, which relies on the combination of the cobalt-mediated polymerization of vinyl acetate and ATRP of styrene. PVAc-CCl3 macroinitiator synthesized from radical and controlled radical telomerization in the presence of CuCl/PMDETA as a catalyst system at 60 °C. 1HNMR, DSC and GPC confirmed a well-defined diblock copolymers microstructures of the reaction initiated with PVAc-CCl3 telomers in the presence of Co(acac)2/DMF. The narrow dispersity indices of PVAc and synthesized diblock copolymers confirmed a well-defined microstructure by initial macroinitiator, implying the effect of living/controlled characteristic of this reaction. The obtained results showed that ATRP of St initiated with PVAc-CCl3 from controlled telomerization (in the presence of Co(acac)2/DMF) grows along with a well-controlled composition and molecular weights as well as the narrow dispersity indices.
References
Tonnar J, Pouget E, Desmazes PL, Boutevin B (2009) Synthesis of poly(vinyl acetate)-block-poly(dimethylsiloxane)-block-poly(vinyl acetate) copolymers by iodine transfer photopolymerization in miniemulsion. Macromol Symp 281:20–30
Tonnar J, Pouget E, Lacroix-Desmazes P, Boutevin B (2008) Synthesis of poly(vinyl acetate)-b-poly(dimethylsiloxane)-b-poly(vinyl acetate) diblock copolymers by iodine transfer polymerization. Eur Polymer J 44:318–328
Semsarzadeh MA, Mirzaei A, Vasheghani-Farahani E, Nekomanesh Haghighi M (2003) Atom transfer radical polymerization of (meth) acrylates and their novel block copolymers with vinyl acetate. Eur Polym J 39:2193–2201
Semsarzadeh MA, Abdollahi M (2009) Atom transfer radical homo- and copolymerization of styrene and methyl acrylate initiated with trichloromethyl-terminated poly(vinyl acetate) macroinitiator: a kinetic study. J Appl Polym Sci 114:2509–2521
Huang CF, Kuo SW, Chen JK, Chang FC (2005) Synthesis and characterization of polystyrene-b-poly(4-vinyl pyridine) block copolymers by atom transfer radical polymerization 12:449–456
Debuigne A, Caille JR, Jerome R (2005) Highly efficient cobalt-mediated radical polymerization of vinyl acetate. Angew Chem Int Ed 44:1101–1104
Semsarzadeh MA, Amiri S (2012) Study of chain sequence in the controlled radical telomerization of vinyl acetate with Co(acac)2 catalyst in bulk. J Polym Res. doi:10.1007/s10965-012-9891-8
Semsarzadeh MA, Amiri S (2012) Controlled free radical polymerization of vinyl acetate with cobalt acetoacetonate. J Chem Sci 124(2):521–527
Jeon HJ, Youk JH (2010) Synthesis of poly(vinyl acetate)-b-polystyrene and poly(vinyl alcohol)-b-polystyrene copolymers by a combination of cobalt-mediated radical polymerization and RAFT polymerization. Macromolecules 43:2184–2189
Semsarzadeh MA, Amiri S (2012) Silicone macroinitiator in atom transfer radical polymerization of styrene and vinyl acetate: synthesis and characterization of pentablock copolymers. J Inorg Organomet Polym. doi:10.1007/s10904-012-9800-y
Debuigne A, Caille JR, Jerome R (2005) Synthesis of end functionalized poly(vinyl acetate) by cobalt mediated radical polymerization. Macromolecules 38:5452–5458
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Semsarzadeh, M.A., Amiri, S. Synthesis and characterization of PSt-b-PVAc diblock copolymers via combination of atom transfer radical polymerization and cobalt-mediated radical polymerization. J Polym Res 20, 139 (2013). https://doi.org/10.1007/s10965-013-0139-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-013-0139-z