Abstract
Cationically photopolymerizable phenyl epoxy-silicone monomers (Ep-Ph-Si) with a high RI and high photopolymerization activity have been synthesized via sequential hydrosilylation reactions of 1,3,5,7- tetramethylcyclotetrasiloxane by attaching first styrene, followed by 4-vinyl-1-cyclohexene-1,2-epoxide in the presence of Lamoreaux catalyst. The content of benzene ring in the monomers was determined by 1HNMR; the epoxy value was examed by chemical titration. The RI of the obtained monomers showed that the benzene ring could improve the refractive indices (RIs) and the RIs of the phenyl epoxy-silicone monomers ranged from 1.50 to 1.52. The cationic photopolymerization of these monomers initiated by triarylsulfonium hexafluorophosphate (P-S) showed that phenyl epoxy-silicone monomers with little amount of benzene ring still exhibited high photopolymerization rate. Those properties are well suitable for the application in UV-curing optical coatings and encapsulation materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cationic polymerizations have attracted increasing attention in recent years due to the growth of applications associated with the synthesis of photoinitiators and monomers [1–4]. The insensitivity toward oxygen of photoinduced cationic polymerization is a great advantage over radical photopolymerization [5–8]. A wide range of different monomer systems, including epoxides, vinyl ethers, oxetanes, and many others, can be employed in cationic polymerization [9–12]. The resulting polymers possess excellent adhesion, chemical resistance and mechanical properties. For these reasons, there is a growing interest in the industrial applications of cationic processes [13–16].
The use of epoxy-silicone monomers and oligomers in cationic photopolymerization is very attractive because epoxy-silicone offers the benefits of both silicone and epoxy resins [17, 18]. The siloxane bond is stable under heat and ultraviolet (UV) light; epoxy resin has a high adhesive strength. The use of silicone resin alone prevents discoloration, but its poor adhesive strength may cause optical delamination between the semiconductor and its encapsulant. Silicone-containing monomers bearing epoxycyclohexane groups have been reported to possess reactivities much higher than those of typical epoxides and exhibit high rates of photopolymerization [19, 20]. Those properties are well suitable for the application in optical coatings, and encapsulation materials for optical devices. However, the low refractive index (RI) of polysiloxane restricts their application in above section. With the increasing demand for high performance, a higher RI is especially important for the optical efficiency and lifetime of such devices [21, 22].
It has been found that introducing aromatic ring can increase the RI [23–25]. Silicone containing phenyl group was usually prepared from phenyl chlorosilane. The reaction process of hydrolyzation reaction of chlorosilane is difficult to control. In this study, the aromatic group styrene was incorporated into tetramethylcyclotetrasiloxane monomer by hydrosilylation reaction and then reacted with the 4-vinyl-1-cyclohexene-1, 2-epoxide (VCHO) in the presence of Lamoreaux catalyst. By changing the ratio of styrene and VCHO in the hydrosilation reaction of tetramethylcyclotetrasiloxane, a series of phenyl epoxy-silicones was obtained. The effects of the contents of benzene ring on the RI were studied. The cationic photopolymerization of these epoxy-silicone monomers was investigated using triarylsulfonium hexafluorophosphate (P-S) as photoinitiators under a high-pressure mercury lamp.
Experimental
Materials
4-Vinyl-1-cyclohexene-1, 2-epoxide (VCHO) and 1,3,5,7- tetramethylcyclotetra- siloxane (D4 H) were chemical reagents purchased from Adrich Chemical and used as received. H2PtCl6·6H2O was purchased from Shanghai Guichun Chemical Material Co.. N-octyl alcohol was purchased from Beijing Chemical Works. The commercial epoxy compounds used in this work was 3,4-epoxycyclohexylmethyl-3, 4-epoxy-cyclohexane carboxylate (ERL4221, epoxy value:0.78) and diglycidyl ether of bisphenol A (E51, epoxy value:0.51), which were industrial products supplied by Dow and Shell Chemical, respectively. Cationic photoinitiator diphenyl-(4-(phenylthio)phenyl)sulfonium hexafluorophosphate (P-S) was synthesized via the condensation of diphenylsufied and diphenylsulfoxide in the MSA-P2O5 [26]. The abbreviations and structures of the epoxy compounds and photoinitiators employed in this study were summarized in Fig. 1. Other reagents and solvents were commercially available and reagent quality.
Instruments
1 H NMR spectra were recorded on a Bruker AV400 unity spectrometer operated at 400 MHz using d6-acetone or CDCl3 as deuterated solvent. FT-IR spectra were recorded on a Nicolet 5700 instrument (Thermo Electron Corporation, Waltham, MA). UV–vis absorption spectra were recorded in CH2Cl2 solution on a Hitachi U-3010 UV–vis spectrophotometer (Hitachi High-Technologies Corporation, Tokyo, Japan). Light intensity was recorded by a UV light radiometer (Photoelectric Instrument Factory, Beijing Normal University, China). The refractive index was measured by WYA-2S ABBE digital refractometer (Shanghai Precision & Scientific instrument Co.LTD., China).
Preparation of Lamoreaux catalyst
Lamoreaux catalyst was self-prepared from H2PtCl6·6H2O and n-octyl alcohol according to the US patent [27]. The catalyst was prepared by dissolving 0.2 g of H2PtCl6·6H2O in 2 g of n-octyl alcohol in a one-port flask and heating the solution at 70 to 75 °C under the electromagnetic stirring for 10 h during which time all the water and hydrogen chloride generated was removed. At the end of this time a product was obtained which was a reddish-brown liquid.
General procedure for the synthesis of phenyl epoxy-silicone monomer (Ep-Ph-Si)
To a 100 ml three-necked round-bottom flask equipped with a reflux condenser, a nitrogen inlet, and a thermometer, 1,3,5,7-tetramethylcyclotetrasiloxane (D4 H; 0.1 mol), styrene (0.1 mol to 0.3 mol), and toluene (10 ml) were loaded. The reaction mixture was refluxed for 1 h. After cooling to room temperature, 2 drops of the Lamoreaux catalyst (1.24 × 10-6 mol Pt) were added. The temperature of the reaction mixture was then raised to 40 °C and the reaction was monitored by thin-layer chromatography (TLC) with petroleum ether as the developing agent. Completion of the reaction was indicated by disappearance of styrene by TLC. The period of reaction was about 6-8 h.
After styrene completely reacted, VCHO (0.1 mol to 0.4 mol) was added dropwise to the mixture, which was then allowed to stand for 6 h at 60 °C. After cooling the reaction mixture to room temperature, 0.0005 g of 2-mercaptobenzothiazole was added to deactivate the catalyst. Toluene and the excess reactant were removed under 60 °C/10 mm Hg, and the product was further purified using a chromatography column to yield a viscous product.
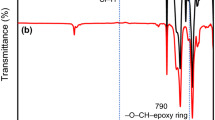
1 H NMR (d6-acetone, 400 MHz) δppm: 7.44-6.81 (m, Ar-H), 4.70 (m, Si-H), 3.06-3.01(m, -O-CH-epoxy ring), 2.85-2.44 (m, Ar-CH2-), 2.12-1.03 (m, -CH2-cyclohexhane ring), 0.99-0.92 (m, Ar-CH-), 0.61-0.54 (m, Si-CH2-), 0.18-0.01 (m, Si-CH3). IR (KBr) ν max (cm−1): 3060 (Ar-H); 2921 (CH3, CH2 and CH); 2121 (Si-H); 1602, 1495 and 1452 (Ar ring); 1257 (CH3-Si); 1078 (Si-O- Si); 905 (−CH-O-CH-epoxy ring).
The yields and epoxy value of the prepared Ep-Ph-Si monomers were shown in Table 1.
Determination of the epoxy value
Exactly 0.5 g of momomer was accurately weighed and placed in a container. A 20 ml solution containing hydrochloric acid (36.5 %, 0.50 ml) and acetone (19.50 ml) was then added. After the monomer dissolved, the container was sealed and placed in the dark for half an hour. Then, 2 or 3 drops of methyl red indicator were added, and the mixture was titrated with a standard sodium hydroxide solution (0.2 mol/L) until the color changed from red to yellow. At the same time, another parallel mixture to the sample and blank was prepared for comparison.
The epoxy value is calculated:
Wherein: V 1-Titration of the blank solution volume of sodium hydroxide required, ml; V 2-Titration of hydrochloric acid in acetone solution by adding epoxy sample required volume of sodium hydroxide, ml; G-Weighed sample mass, g; M-Concentration of standard sodium hydroxide solution, mol/L.
Photopolymerization procedure
0.01 g of photoinitiator was added to 1.00 g of epoxide and then stirred. The formulated mixture was then cast on a glass plate to a thickness of about 100 μm and irradiated by a high-pressure mercury lamp. The lamp was placed over the plate with distance of 10 cm. After different time's irradiation, the glass plate along with the mixture was separately weighed (W 1 ) and then was immersed into anhydrous alcohol/acetone for about 30 min to remove uncured compositions. After removing from the anhydrous alcohol/acetone, the glass plate with the cured resin was dried in oven and was weighed (W 2 ). The gel yield (%) was calculated according to the following equation:
where W 0 is the weight of the glass plate (g).
Test for light transmission of cured epoxy-silicone resin
The light transmittance of cured epoxy-silicone resin was measured on 2 mm thick specimens over the range of 300 to 800 nm by a UV–vis spectrophotometer. The specimens of cured epoxy-silicone resin were obtained from the mixture of 0.02 g P-S, 0.8 g Ep-Ph-Si monomer and 0.2 g ERL4221 irradiated for 10 min with a high-pressure mercury lamp.
Results and discussion
Synthesis and characterization of monomers
Phenyl epoxy-silicone monomers were synthesized by the well-known hydrosilylation reaction. The synthetic process is depicted in Scheme 1. In the first step, the hydrosilylation reaction of D4 H and styrene occurred, catalyzed by Lamoreaux catalyst at a certain Si-H to double bond ratio. In the next stage, the remaining reactive Si-H of the first obtained product reacted with VCHO. In the hydrosilylation reaction, it was found that styrene is more active than VCHO. So, the most preferred reaction temperature of D4 H and styrene was 40 °C and the most preferred reaction temperature of D4 H and VCHO was 60 °C. Another condition that was considered was that a long reaction time results in the partial ring-open polymerization of the epoxy group catalyzed by Lamoreaux catalyst and gelation occurs. Thus, the optimal reaction time was less than 8 h.
The compositions of the epoxy-silicone monomers were determined by 1 H NMR. The 1 H NMR spectrum of Ep-Ph-Si-2 is shown in Fig. 2. The peaks at 7.44-6.81 ppm belong to the hydrogen in the benzene ring and the area of the peaks is S3. The peaks at 4.70 ppm belong to the left Si-H and the area of the peaks is S4. The peaks at 3.06-3.01 ppm belong to hydrogen in the epoxy ring and the area of the peaks is S5. The average numbers of blocks are given below: the average number of the left Si-H in Ep-Ph-Si is a; the average number of the benzene group in Ep-Ph-Si is b+c (a mixture of two isomers: α-adduct and β-adduct); the average number of the epoxy group in Ep-Ph-Si is d. Based on four Si-H bonds in one D4 H molecule, the average numbers of a, b+c, and d in Ep-Cz-Si-1 was calculated as follows:
The composition of the prepared epoxy-silicone monomers was also shown in Scheme 1.
The refractive index of monomers and cured epoxy-silicone resins
The current study focused on introducing different proportions of benzene ring into D4H; consequently, different RIs of the phenyl epoxy-silicone monomers were obtained. The RI of the obtained monomers was measured by a digital refractometer at 20 °C, as shown in Table 1. Figure 3 shows the RI of Ep-Ph-Si varying with the ratio of Si-C bond. The ratio of Si-C bond is the mole ratio of Si atom to phenyl group in Ep-Ph-Si and was calculated calculated as follows:
The RI of Ep-Si is 1.495 at 20 °C and is higher than that of D4 H (1.389). This result indicates that the epoxy group can improve the refractivity of the organosilicon materials. Though the epoxy value of Ep-Si is highest, the order of the RIs is Ep-Ph-Si-3 >Ep-Ph-Si-2 > Ep-Ph-Si-1 > Ep-Si. It can be concluded that benzene ring is more effective than epoxy group in improving the refractivity of the organosilicon materials. More benzene ring content results in a higher RI.
The RI values of the cured resins after photo-polymerization have been measured. The data were listed in Table 1. It can be seen that the RI values of the cured resin after photo-polymerization were a little higher than that of monomer before photo-polymerization.
The light transmittance of cured epoxy-silicone resin was measured over the range of 300 to 800 nm by a UV–vis spectrophotometer. The specimens of cured epoxy-silicone resin were obtained from the mixture of 2 wt% P-S, 80 % Ep-Ph-Si monomer and 20 % ERL4221 irradiated with a high-pressure mercury lamp. Change of optical transmittance at different wavelength is shown in Fig. 4 for the cured epoxy-silicone. The curves in Fig. 4 show that all the cured epoxy-silicone specimens have good light transmittance upon 400 nm. The light transmittance of specimens from Ep-Si is better than those of specimens from Ep-Ph-Si.
Cationic photoinitiated polymerization of the monomers
As aforementioned, there is considerable interest in cationically photopolymerization activities of epoxy-silicone monomers. Therefore, the photopolymerization rates of the phenyl epoxy-silicone monomers were evaluate to determine whether relationships between their structures and reactivities could be established. For this purpose, the gel yield method was employed.
The photopolymerization of the epoxy-silicone monomers was carried out under a high-pressure mercury lamp with 1 mw/cm2 of light intensity. P-S was used as cationic photoinitiators.
P-S is an important cationic photoinitiator in photopolymerization irradiated by a high-pressure mercury lamp because it has some absorption above 300 nm. However, it was found to show poor solubility in the synthesized phenyl epoxy-silicone monomers. Thus, in the study of the photopolymerization of Ep-Ph-Si, ERL4221 and E51 were mixed with Ep-Ph-Si at a weight ratio of 1:2 to improve the solubility of the photoinitiator. At the same time, monomer ERL4221 and oligomer E51 served as the model monomer that provided baseline reactivity to measure any effect on the polymerization rate due to the incorporation of the phenyl ring. The resulting data were averaged and the curves of the gel yield vs. time are presented.
Figure 5 shows the gel yield curves of the photopolymerization of Ep-Ph-Si mixed with ERL4221 using 3 wt% P-S as the photoinitiator. For comparison, the gel yield curves of the photopolymerization of ERL4221 are also shown in Fig. 5. The order of the polymerization rates is ERL4221 + Ep-Si and ERL4221 + Ep-Ph-Si-1 > ERL4221 + Ep-Ph-Si-2 > ERL4221 > ERL4221 + Ep-Ph-Si-3. The order of the photopolymerization rates of Ep-Ph-Si is consistent with the order of their epoxy value. It was worth to point that the photopolymerization activities of Ep-Si, Ep-Ph-Si-1 and Ep-Ph-Si-2 are higher than that of ERL4221, though their epoxy values are lower than that of ERL4221. Crivello et al. have proposed that there are two major contributory factors to the outstanding reactivity of epoxy-silicone monomers, including the inherent ring strain and the absence of other basic groups in the epoxycyclohexane ring system.
Figure 6 shows the gel yield curves of the photopolymerization of Ep-Ph-Si mixed with E51 using 3 wt% P-S as the photoinitiator. When Ep-Si and Ep-Ph-Si-1 were mixed with E51, the photopolymerization rate of the mixtures is higher than that of E51; When Ep-Ph-Si-2 and Ep-Ph-Si-3 were mixed with E51, the photopolymerization rate of the mixtures is lower than that of E51.
From the above disccusion, it can be found that phenyl epoxy-silicone monomers with little amount of benzene ring still exhibited high photopolymerization rate. However, with the increasement of the contents of benzene ring, the epoxy value of epoxy-silicon monomers decrease and the photopolymerization activities decrease.
Conclusion
Three Ep-Ph-Sis and a Ep-Si monomers were synthesized by hydrosilylation reaction in the presence of Lamoreaux catalyst. RI measurements reveal that both the phenyl group and epoxy group can improve the RI of the epoxy-silicone monomers. The order of the RIs is Ep-Ph-Si-3 >Ep-Ph-Si-2 > Ep-Ph-Si-1 > Ep-Si. Benzene ring is more effective than epoxy group in improving the refractivity of the organosilicon materials. The study on the cationic photopolymerization of Ep-Ph-Si under a high-pressure mercury lamp reveals that phenyl epoxy-silicone monomers with little amount of benzene ring still exhibited high photopolymerization rate.
References
Yagci Y, Jockusch S, Turro NJ (2010) Macromolecules 43:6245–6260
Tehfe MA, Lalev J, Gigme DS, Fouassier JP (2010) Macromolecules 43:1364–1370
Crivello JV, Ma J, Jiang F, Hua H, Ahn J, Acosta Ortiz R (2004) Macromol Symp 215:165–177
Zhan F, Cheng X, Shi WF (2012) Polymer Adv Tech 23:645–651
Tehfe MA, Lalevéel J, Gigmes D, Fouassier JP (2010) J Polymer Sci A: Polymer Chem 48:1830–1837
Chen Y, Li GL, Zhang HQ, Wang T (2011) J Polymer Res 18(6):1425–1429
Morita Y, Tajima S, Suzuki H, Sugino H (2008) J Appl Polymer Sci 109:1808–1813
Jianxin F, Linjie Z, Chunmeng L, Shuli T, MingWan Y, Costas G (2009) Polymer Eng Sci 49:1107–1116
Lalevée J, Dumur F, Mayer CR, Gigmes D, Nasr G, Tehfe MA, Telitel S, Savary FM, Graff B, Fouassier JP (2012) Macromolecules 45:4134–4141
Kruger K, Tauer K, Yagci Y, Moszner N (2011) Macromolecules 44:9539–9549
Durmaz YY, Kukut M, Monszner N, Yagci Y (2009) Macromolecules 42:2899–2902
Liow SS, Lipik VT, Widjaja LK, Abadie MJM (2012) J Polymer Res 19:9748. doi:10.1007/s10965-011-9748-6
He JW, Liu F, Vallittu PK, Lassila LVJ (2012) J Polymer Res 19(8):9932. doi:10.1007/s10965-012-9932-3
Sangermano M, Tonin M, Yagci Y (2010) Eur Polym J 46:254–259
Tehfe MA, Lalev J, Savary FM, Graff B, Fouassier JP (2011) Macromolecules 44:8374–8379
Ortiz RA, Sangermano M, Bongiovanni R (2006) Prog Org Coat 57:159–164
Wang WJ, Perng LH, Hsiue GH, Chang FC (2000) Polymer 41:6113–6122
Jang M, Crivello JV (2003) J Polymer Sci A: Polymer Chem 41:3056–3073
Crivello JV, Lee JL (1990) J Polymer Sci A: Polymer Chem 28:479–503
Crivello JV (1994) J Polymer Sci A: Polymer Chem 32:683–697
Lin YH, You JP, Lin YC, Tran NT, Shi FG (2010) IEEE Trans Compon Packag Tech 33:761–766
Yang SC, Kim JS, Jin JH, Kwak SY, Bae BS (2011) J Appl Polymer Sci 122:2478–2485
Chen ZD, Deng XS, Peng YB (2010) New Chem Mater 38:49–51
Kim JS, Yang SC, Bae BS (2010) Chem Mater 22:3549–3555
McGrath JE, Rasmussen L, Shultz AR (2006) Polymer 47:4042–4057
Akhtar SR, Crivello JV, Lee JL (1990) J Org Chem 55:4222–4225
Lamoureaux FH, U.S. Patent 3,220,972, (1965)
Acknowledgements
The authors wish to thank for financial support of National High Technology Research and Development Program 863 (No. 2011AA03A109).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, H., Zhang, X.S., Li, Y.J. et al. Synthesis and cationic photopolymerization of phenyl epoxy-silicone monomers. J Polym Res 19, 19 (2012). https://doi.org/10.1007/s10965-012-0019-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-012-0019-y