Abstract
Visible light curing of diglycidyl ether of bisphenol-A (DGEBA) epoxy oligomer and acrylate monomers photoinitiated by (η6-benzophenone)(η5-cyclopentadienyl) iron hexafluorophosphate (Fc-BP) under a halogen lamp were studied by near infrared spectroscopy. Fc-BP exhibited high efficiency in the radical photopolymerization of acrylate monomers, even without the presence of tertiary amines. Under the same light source, however, benzophenone did not show any photoinitiating ability. Fc-BP could also be used to photoinitiate the cationic polymerization of DGEBA. There was an obvious increase in the photopolymerization rate of DGEBA and a decrease in the induction period when benzoyl peroxide was used as a photosensitizer. The induction period at the beginning of DGEBA cationic polymerization was eliminated by introducing a certain amount of cycloaliphatic epoxy monomer ERL4221 as an active diluent. However, the final epoxy conversion was decreased when ERL4221 was used.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Photointiators for visible light curing of photopolymerizable formulations are still a challenge due to the high demands of modern applications, such as dental filling materials, reprography (e.g., photoresists, printing plates, integrated circuits), laser-induced 3D curing, holographic recordings, and nanoscale micromechanics [1–3]. Whereas several radical photoinitiators, such as 2, 4, 6-trimethyl benzoyldiphenylphosphine oxide, cleavable titanocene and camphorquinone, are commercially available and highly reactive [4–6], there are very few cationic photoinitiators available for visible light curing.
Since its absorption tails out above 400 nm, ferrocenium salts are attractive for cationic polymerization under visible light (400–700 nm) [7, 8]. A ferrocenium salt with a benzophenone moiety, (η6-benzophenone)(η5-cyclopentadienyl) iron hexafluorophosphate (Fc-BP), has recently been developed as novel photoinitiators in our laboratory [9, 10]. Its cationic photoinitiating ability in the photopolymerization of cycloaliphatic epoxy monomers has been studied, and it has been found effective as a novel radical photoinitiator in the visible light curing of acrylates. Fc-BP can be used in the halogen lamp (370–520 nm). In contrast, benzophenone is one of the most widely used radical photoinitiators, but it has nearly no absorption above 300 nm.
Although the cationic photopolymerizations of cycloaliphatic epoxy monomers have been widely reported, little information can be found in literature regarding the photopolymerization of the diglycidyl ether of bisphenol-A (DGEBA) epoxy oligomer, which are widely used in thermal polymerization for its good mechanical and thermal properties, high resistance to solvents and corrosive agents, outstanding adhesion to various substrates, and low shrinkage upon curing. Therefore, one of the aims of this study is to explore the photopolymerization of the DGEBA epoxy oligomer cured using Fc-BP under visible light.
The most widely used techniques to assess the extent of polymerization in composites have been the determination of surface hardness, measurement of the heat of reaction liberated during polymerization by differential scanning calorimetry (photo-DSC), and direct chemical analysis of conversion by real time infrared (RT-IR) spectroscopy, including mid-infrared (mid-IR) and near-IR (NIR) spectroscopy [11, 12]. In this study, near-IR spectroscopy (NIR) is employed to investigate the polymerization process of epoxy oligomer and acrylates using Fc-BP, in view of the superior sensitivity of this technique for monitoring polar groups.
Experimental
Materials
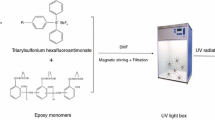
The epoxy oligomer, DGEBA, was obtained from Wuxi Resin Factory (Wuxi, China). The epoxy equivalent weight of DGEBA was 227 g/mol. The epoxy monomer, 3,4-epoxycyclohexylmethyl-3,4-epoxycyclohexane carboxylate (ERL4221), was obtained from Tianjin Synthetic Material Research Institute (Tianjin, China). The monomers polymerized through a radical mechanism were hydroxyethyl acrylate (HEA), trihydroxymethylpropyl triacrylate (TMPTA), and tripropylene glycol diacrylate (TPGDA). TMPTA and TPGDA were obtained from Sartomer (Warrington, PA). HEA was purchased from Sinopharm Group Chemical Reagent Co. (Beijing, China). Scheme 1 summarizes the abbreviations and structures of the compounds employed in this study.
Instruments
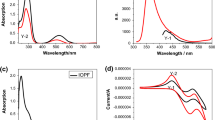
UV-Vis absorption spectra were recorded in MeCN solution on a UV-Vis spectrophotometer (Hitachi U-3010, Hitachi High-Technologies Corporation, Tokyo, Japan). Light intensity was recorded by a UV light radiometer (Photoelectric Instrument Factory, Beijing Normal University, China).
NIR spectroscopy measurement
In the present work, near infrared (NIR) spectroscopy was used to measure the double bond and epoxy group conversion in the resins as a function of exposure time. All samples were photocured in 1.8 mm thick plastic molds with a 10 mm diameter center. The molds were clamped between two glass slides. The samples were irradiated with a visible light source (λ = 370–520 nm, halogen lamp, 50 W). For each sample, the RT-NIR runs were repeated three times. The specimens were irradiated at different time intervals by manually controlling the curing light. Upon collection of the uncured resin NIR spectrum, spectra were obtained immediately after each exposure interval. The double bond conversion profiles were calculated from the decay of the absorption band located at 6,165 cm−1 as described by Stansbury and Dickens [13]. The epoxy peak at 6,075 cm−1 was used to calculate the epoxy conversion [14].
The epoxy conversion was calculated by Formula (1):
where ST is the area of the epoxy C-H characteristic absorbance peak, S0 is the initial area of the epoxy C-H characteristic absorbance peak, RT is the Ts area of the reference peak, and R0 is the initial area of the reference peak.
The double bond conversion was calculated by Formula (2):
where ST is the area of the C=C characteristic absorbance peak and S0 is the initial area of the C=C characteristic absorbance peak.
Results and discussion
Measurement of conversion with NIR
The NIR region, which extends from about 14,000 to 4,000 cm−1, encompasses bands that result from the harmonic overtones of fundamental and combination bands associated with hydrogen atoms. This is why compounds containing O–H, N–H, and/or C–H bonds lend themselves favorably to NIR spectroscopic analysis. In some recent reports, NIR spectroscopy has been applied to the characterization of the thermal curing of DGEBA and in the photopolymerization of acrylate monomers. The NIR range has one major advantage over the MIR range in that it (NIR) gives bulk monomer conversion data on sample dimensions that are relevant to practice. An advantage that further favors the NIR method is that glass has a very weak absorption at approximately 4,500 cm−1, so that the spectra can be obtained through glass substrates.
In this work, NIR spectroscopy was used to measure epoxy and acrylate conversion as a function of photoexposure time. The NIR spectra of DGEBA epoxy and TPGDA acrylate monomer photoinitiated by Fc-B1 were obtained after irradiation with a halogen lamp at different exposure times (Fig. 1). The peak at 6,075 cm−1 results from the first overtones of the fundamental epoxy C-H stretch. The peak at 4,670 cm−1, which corresponds to the –CH stretching vibration due to benzene ring, was used as a reference. The peak at 6,165 cm−1 results from the first overtones of the fundamental C-H stretch of acrylate. With an increase in exposure time, changes to the spectra were observed, and the corresponding absorption bands of epoxy and acrylate were gradually lowered.
Radical photopolymerization of acrylate monomers photoinitiated by Fc-BP
Acrylate monomers irradiated under a halogen lamp were photopolymerized with 1% Fc-BP. The acrylate monomers HEA, TMPTA, and TPGDA, which are mono-, di-, and tri-functional acrylate monomers, respectively, were chosen as the model monomers for the radical photopolymerization. The light intensity on the surface of the samples was 1 mW/cm2. The double bond conversion versus irradiation time curves for the radical polymerization of the acrylate monomers is shown in Fig. 2.
The results showed that Fc-BP could rapidly photoinitiate acrylate polymerization under a halogen lamp. The double conversion of HEA could reach 75% within 20 s of irradiation. The higher the functionality degree of the acrylate monomer was, the lower the final functional group conversion. The photocrosslinking of the unreacted monomers were inhibited by the polymers. Hence, the final double bond conversion of TMPTA was the lowest among the three acrylate monomers.
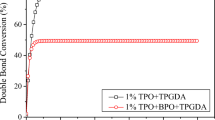
In this study, the effect of several kinds of amines, such as triethylamine, triethanolamine, and N,N-dimethylaniline, on the polymerization of acrylate monomers irradiated by Fc-BP was also investigated (Fig. 3). Adding amines did not increase the photoinitiating activity. It is believed that the effects of the addition of BPO and amines on the double bond conversion of acrylates are closely related to the mechanism of radical photoinitiation of ferrocenium salt. There are mainly two different mechanisms for free radical photopolymerization: one is the fracture type and the other is the hydrogen atom abstraction type. For a single benzophenone, as a classical hydrogen atom abstraction type initiator, the introduction of tertiary amines commonly will increase the double bond conversion of acrylates. However, the results showed that the addition of BPO and amines lowered the double bond conversion of acrylates, so the mechanism of free radical polymerization photoinitiated by the novel Fc-BP may be different from that of a single benzophenone [15].
Cationic photopolymerization of DGEBA
The epoxy conversion versus irradiation time curves for the cationic polymerization of DGEBA photoinduced by 2.0 wt% Fc-BP in the presence and absence of the photosensitizer benzoyl peroxide (BPO) are shown in Fig. 4. The light intensity on the surface of the samples was 30 mW/cm2. The results showed that Fc-BP were able to effectively photoinitiate the polymerization of DGEBA under a halogen lamp. The induction period at the beginning of the DGEBA cationic polymerization, however, was relatively long.
From Fig. 4, it can be seen that there was an obvious increase in the photopolymerization rate of DGEBA and a decrease in the induction period in the presence of 1 wt% BPO compared to when there was an absence of BPO. The photoinitiated and photosensitized mechanisms of epoxide irradiated by ferrocenium salts have been reported in previous papers.
The cycloaliphatic epoxy monomers are more reactive than the DGEBA oligomers in the photopolymerization reactions. Therefore, cycloaliphatic epoxy monomers are often used as active diluents in the photopolymerization of DGEBA. The effect of introducing the reactive diluent ERL4221 on the conversion of DGEBA to an Fc-BP photoinitiated polymer was also studied. Cycloaliphatic epoxy monomer do not have characteristic peaks that decrease with irradiation time in NIR. Thus, NIR spectroscopy was used to measure only the epoxy conversion of DGEBA as a function of exposure time in the DGEBA+ERL4221 system (Fig. 5). In Fig. 5, the induction period at the beginning of the DGEBA cationic polymerization was eliminated by introducing a certain amount of ERL4221. However, the addition of ERL4221 suppressed the epoxy conversion and the amount of ERL4221 added did not have clear trend with the conversion. Our explanation is that the ERL4221 is a good reactive diluent, which is able to shorten the induction period of the cationic polymerization of the DGEBA system, and its polymerization rate is obviously faster than that of the DGEBA oligomers. Hence, ERL4221 polymerizes first when the DGEBA/ERL4221 system is irradiated, which may block the later crosslinking of the DGEBA oligomers regardless of the amount of ERL4221, leading to a suppression of the epoxy conversion as observed by NIR results in Fig. 5.
Conclusion
Fc-BP exhibited high efficiency in the radical photopolymerization of acrylate monomers under a halogen lamp. The mechanism of photoinitiation was different from that of a single benzophenone. Fc-BP could also photoinitiate the photocuring of DGEBA. The induction period at the beginning of the DGEBA cationic polymerization was eliminated by introducing a certain amount of ERL4221 epoxy monomer.
References
Crivello JV (1998) In: Bradley G (ed) Photoinitiators for free radical, cationic and anionic photopolymerization, 2nd edn. New York, Wiley
Fouassier JP, Allonas X, Burget D (2003) Photopolymerization reactions under visible lights: principle, mechanisms and examples of applications. Prog Org Coat 47:16
Kawamura K, Kato K (2004) Synthesis and evaluation as a visible-light polymerization photoinitiator of a new dye-linked bis(trichloromethyl)-1, 3, 5-triazine. Polym Adv Technol 15:324
Guggenberger R, Lechner G, Weinmann W (1997) Oxides as photoinitiators in dental materials. Am Chem Soc Div Polym Chem Polym Prepr 38:105
Jakubiak J, Allonas X, Fouassier JP, Sionkowska A, Andrzejewska E, Linden LA, Rabek JF (2003) Camphorquinone–amines photoinitating systems for the initiation of free radical polymerization. Polymer 44:5219
Ganster B, Fischer UK, Moszner N, Liska R (2008) New photocleavable structures, 4: acylgermane-based photoinitiator for visible light curing. Macromol Rapid Commun 29:57
Meier K, Zweifel H (1986) Curable composition and it use. J Image Sci 30:174
Zweifel H, Meier K (1985) Imaging with cationic organomftallic photoinitiators. Am Chem Soc Div Polym Chem 26:347
Wang T, Chen JW, Li ZQ, Wan PY (2007) Several ferrocenium salts as efficient photoinitiators and thermal initiators for cationic epoxy polymerization. J Photochem Photobiol A Chem 187:389
Wang T, Li ZQ, Zhang Y, Hassan K, Wang XN (2009) (η6-N-alkylcarbazole) (η5-cyclopentadienyl) iron hexafluorophosphate salts in photoinitiated and thermal epoxy polymerization. Polym Eng Sci 49:613
Billaud C, Vandeuren M, Legras R, Carlier V (2002) Quantitative analysis of epoxy resin cure reaction: a study by near-infrared spectroscopy. Appl Spectrosc 56:1413
Kortaberria G, Arruti P, Gabilondo N, Mondragon I (2004) Curing of an epoxy resin modified with poly(methylmethacrylate) monitored by simultaneous dielectric/near infrared spectroscopies. Eur Polym J 40:129
Stansburya JW, Dickens SH (2001) Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent Mater 17:71
Jiang B, Huang YD (2008) Quality inspection of laid fabric epoxy resins prepreg by near infrared spectroscopy. Compos A 39:712
Hoyle CE, Viswanathan K, Clark SC, Miller CW, Nguyen C, Jonsson S, Shao L (1999) Sensitized polymerization of an acrylate/maleimide system. Macromolecules 32:2793
Acknowledgements
The authors wish to thank for financial support from Beijing University of Chemical Technology (Project Grant No. 00620350).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Li, G., Zhang, H. et al. Visible light curing of bisphenol-A epoxides and acrylates photoinitiated by (η6-benzophenone)(η5-cyclopentadienyl) iron hexafluorophosphate. J Polym Res 18, 1425–1429 (2011). https://doi.org/10.1007/s10965-010-9547-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-010-9547-5