Abstract
Starting from MPEG-NH2, a series of amphiphilic triblock copolymers MPEG-b-PLL-b-PLA were synthesized through PEG-NH2-initiated ring-open polymerization of N ε-benzyloxycarbonyl-L-lysine, followed by acylation coupling between the amino-terminated MPEG-b-PZLL-NH2 and carboxyl-terminal PLA and the deprotection of amines. The block copolymers were characterized by FT-IR, 1H NMR, GPC, DSC and TEM. The copolymer functional groups, molecular and block structures were verified by FT-IR, 1H NMR and DSC, respectively. The GPC results indicate that the chain lengths of each block can be controlled by varying the feed ratios of the monomer and initiator, providing the polymer samples with a narrow molecular weight distribution (M w /M n = 1.10 ~ 1.25). The TEM analysis shows that the triblock polymers can self-assemble into polymeric micelles in aqueous solution with spherical morphology. The cell-cytotoxicity assay indicates that the triblock polymers show no obvious cytotoxicity against Bel7402 human hepatoma cells.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The discovery and delivery of antitumor drugs have remained a major goal in the past decades [1]. The development of each single-component antitumor drug succeeds in a limited level because of in vivo multidrug resistance [2]. The hope of drug delivery systems to bypass the multidrug resistance probably lies on the combinatorial therapeutics of multiple drugs involving different pharmacological mechanisms before the mechanisms of multidrug resistance have been completely identified. The majority of antitumor drugs discovered by the pharmaceutical industry today are hydrophobic compounds [3]. Poor solubility and hydrophobic character of drugs limit their successful applications in cancer therapy. Relative to highly soluble compounds, low drug solubility often causes in vivo consequences in systematical administration including lowered bioavailability, incomplete release from the dosage form, and higher personalized variability. Therefore, novel biocompatible carriers for hydrophobic antitumor drugs remain to be determined in the long term until the actualization of a technologically-mature drug delivery system.
Among the nonviral gene delivery carriers, poly(amino acid)s (PAA) are very important biological macromolecules due to their excellent physical properties, biocompatibility and biodegradability, which are suitable for biomedical applications such as sutures, artificial tissues implants and drug delivery [4–6]. PAA usually serves as the hydrophilic or hydrophobic segment in these amphiphilic copolymers because they may be acted as nanocarriers for either hydrophilic or hydrophobic drugs depending on the nature of amino acids used as building blocks [7, 8]. In special cases both hydrophilic and hydrophobic segments are made of PAAs [9, 10]. Poly(L-lysine) (PLL) is a kind of cationic poly(amino acid) that possesses side chains with terminal amines with the ability to deliver DNA molecules and siRNA, the low immunogenicity and relative safety [11–14]. It is an attractive option for gene therapy techniques since it is synthesized from a low cytotoxic, naturally occurring monomer [14]. Furthermore, the composition of PLL is definite so that it can be prepared in large quantities with relative ease. At the same time, it is possible to design and develop new ideal vector systems because of the diversity of the chemical structure of PLL [15]. Therefore, chemical modifications of aliphatic polyesters can be realized by preparing hyperbranched polyesters [16, 17], star block copolymers [18] and preparing co-polyesters [19] such as PEG-polyester and polyester-poly(amino acid) [20, 21].
Poly(L-lactic acid) (PLA) has been utilized as an important synthetic biodegradable material in the medical and pharmaceutical fields [22–26]. Such fields include surgical repair carriers, in drug delivery and temporary matrixes or scaffolds [27–29] in tissue engineering [30] because of its good biocompatibility, high mechanical properties and excellent shaping and molding properties [31, 32]. However, PLA cannot be easily modified with biologically active moieties due to lack of highly reactive functional group as a trigger of chemical reaction on the hydrophobic surface of the PLA matrix. Therefore, the application scope of poly(L-lactide) is limited. To address these issues described above, the introduction of many kinds of hydrophilic units into PLA has been tried to improve the property of hydrophobic aliphatic PLA. The techniques of copolymerization of L-lactide with other monomers and chemical modification on the poly(L-lactide) chain directly have been investigated [33–35].Additionally, poly(ethylene glycol) (PEG) is a kind of polyether approved by FDA for in vivo experiments and it has been widely used in the preparation of proteins, polypeptides, enzymes and other new bio-medical materials because of its biocompatibility, hydrophilicity, low cytotoxicity and nonimmunogenicity. With respect to block copolymers containing polyesters, many kinds of hydrophilic units, such as PEG-polyester and polyester-poly(amino acid) have been incorporated into PLA and the resulting copolymer has been investigated in a number of medical and pharmaceutical applications [23, 24]. Recently many investigations have attempted to provide functionalization of the polyester and therefore improve the hydrophilicity [36].
Through the synthesis of the block copolymer consisting of PEG and poly(amino acid) and PEG-PZLL which is the intermediates of this study were once reported, [37, 38] the multiple sequential combination of PEG, PLA and PLL, such as PEG-PLA-PLL [14, 38–40] and PLA-PLL-PEG [41] could yield attractive block copolymers for drug delivery. The investigation of these copolymers starting from PEG-OH, PEG-NH2, or PLA-NH2 remains a highly desirable issue for the following drug delivery.
Recently a topic of great interest has been focused on the formation of polymeric micelles by the self-association of block copolymers consisting of hydrophilic and hydrophobic segments in aqueous medium currently [42–46]. At present the laboratory preparation of polymer micelles is usually carried out in two steps, firstly dissolving the polymer in an organic solvent and then inducing aggregation by the addition of water [47]. If the obtained polymeric micelles exhibited no obvious cytotoxicity against human cells so they should be useful for biodegradable biomedical materials such as drug and gene delivery vehicles.
Here, we propose a dual drug delivery strategy named cocktail therapeutics, that is, a nanocarrier that is able to load simultaneously antitumor chemical therapeutical agents (such as Paclitaxel) and oligonucleotides (such as siRNA), which involves two different pharmacological mechanisms. In this respect, we have designed and synthesized an amphiphilic triblock copolymer, poly(ethylene glycol)-b-poly(L-lysine)-b-poly(L-lactide) (PEG-b-PLL-b-PLA), which is expected to self-assemble into spherical nanocarriers with a core-corona-shell sandwich structure by the solvent diffusion method. The solvents used for the micelle preparation of the triblock copolymers are DMF and water. The self-assembly behavior of the triblock copolymer with PEG and PLL as the hydrophilic block and PLA and PZLL as hydrophobic blocks was investigated by TEM. The PEG shell contributes to the hydrophilic (or water soluble) nature and biocompatibility of drug nanocarriers; PLL interlayer containing amino groups allows for the loading of DNA or RNA therapeutical agents by polyion complex; PLA core is one of the certificated candidates for hydrophobic antitumor therapeutical agents, such as Paclitaxel.
Experimental
Materials
N ε- benzyloxycarbonyl L-lysine [Lys(Z)] was purchased from GL Biochem (Shanghai) Co. Ltd. and used without further purification. Triphosgene was purchased from TCI Co. Ltd. Japan and used without further purification. N ε-benzyloxycarbonyl-L-lysine N-carboxy anhydride [lys(Z)-NCA] was prepared in 89.5% yield according to the literature method reported by Poché et al. [48]. and Dorman et al. [49]. using bis(trichloromethyl)carbonate (triphosgene) [50]. Methoxypolyethyleneglycol (MPEG, M n = 5000) was purchased from Shearwater Polymers. Carboxyl-terminal poly (L-lactic acid) (M n = 5000, M n /M w = 1.25) was purchased from Daigang (Jinan) biological technology Co. Ltd. and used without further purification. 33% HBr/AcOH from Sigma-Aldrich was used without further purification. Tetrahydrofuran(THF) and n-hexane were dried and distilled in the presence of sodium and benzophenone before use. N, N-Dimethylformamide (DMF) was purified by distillation from CaH2 under reduced pressure and subsequently stored over molecular sieves (4 Å) under argon atmosphere before use. Methylene chloride and chloroform (Wako Pure Chemical) were purified by distillation over CaH2 and stored over molecular sieves (4 Å) before use. Pyridine was purified by distillation from KOH (pellets) and stored in a flask sealed with paraffin wax before use. Trifluoroacetic acid (TFA) was purchased from Wako Pure Chemicals and was used without further purification. Dicyclohexylcarbodiimide(DCC), N-hydroxy succinimide (NHS), sodium azide (NaN3), p-Toluenesulfonyl chloride (TsCl), triphenylphosphine, acetonitrile were purchased from commercial suppliers and used as received without further purification.
MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) (Invitrogen), DMSO (Invitrogen), Hela human cervical cancer cells (ATCC), Bel7402 human hepatoma cells (ATCC), PBS(Phosphate Buffered Saline) (Invitrogen), RPMI-1640 complete medium (containing 10% newborn calf serum) (Invitrogen), Tripsin 1:250 (Amresco),96-well plate (Invitrogen) were used as received. Microplate Reader (Thermo Fisher Scientific) was used for MTT assay.
Characterization
FT-IR spectra in pressed KBr pellets were recorded using a fourier transformation infrared spectrometer (WQF-410). 1H NMR spectra in D2O, DMSO-d 6 or CDCl3 were measured with a Varian INOVA-400 nuclear-magnetic resonance spectrometer at room temperature (20 °C), chemical shifts are reported in parts per million (ppm), and tetramethylsilane was used as an internal standard. Gel permeation chromatography (GPC) was performed using a chromatograph to detect the molecular weights and molecular weight distributions of the polymers. The molecular weights and molecular weight distributions of EZL and EZLA were measured on a PL-50 GPC equipped with two PL gel 5 μm MIXED-C columns connected in series and an internal refractive index (RI) detector. The columns were eluted with DMF containing lithium bromide (0.05 M) at a flow rate of 0.7 mL/min at 48 °C. The molecular weights were calibrated with poly (ethylene glyco1) standards. The molecular weights and molecular weight distributions of ELA were measured on a PL-50 GPC equipped with two PL aquagel-OH 30 8 μm and 40 8 μm columns connected in series and an internal RI detector. The columns were eluted with H2O containing sodium chloride (0.1 M) at a flow rate of 1.0 mL/min and were maintained at a temperature of 40 °C. The molecular weights were calibrated with poly (ethylene glyco1) standards. Differential scanning calorimetry (DSC) was recorded on NETZSCH STA 409 PC/PG instruments with N2 at a rate of 50 mL/min. The samples were scanned from 20 to 300 °C at a rate of 10 °C /min. Dynamic light scattering (DLS) was performed using a Malvern MPT-2 multi purpose titrator Zeta potentiometer (Malvern, UK) to determined the mean size of nanoparticles. The solution of nanoparticles was performed at a scattering angle of 90°and at 25 °C. Transmission electron microscopy (TEM) was performed using a JEOL-1230 operating at an acceleration voltage of 100 kV.
MPEG-OTS, MPEG-N3 and MPEG-NH2 were prepared in 38%, 91% and 87% yield, respectively, by following the procedure reported in supporting information of ref. (51).
Synthesis of diblock copolymer MPEG-b-PZLL-NH2 (EZL)
MPEG-NH2 was added to 40 mL CHCl3 as the macroinitiator at a monomer/initiator ratio of 50:1 (EZL1), 100:1 (EZL2), 150:1 (EZL3), 200:1 (EZL4), 250:1 (EZL5) and 300:1 (EZL6). The detailed feeding amount of material for MPEG-b-PZLL-NH2 synthesis is presented in Table 1. The mixture solution was stirred under an inert atmosphere for 72 h at 40 °C. The product mixture was precipitated with an excess of anhydrous ethanol under vigorous stirring to obtain a white solid, while the unreacted MPEG-NH2 remained dissolved in the mixture solution. After filtration, diblock copolymer MPEG-b-PZLL-NH2 (EZL) was obtained and subsequently dried under reduced pressure at 40 °C for 24 h. All the purified yields were 79.5–85.0%.
Synthesis of triblock copolymer MPEG-b-PZLL-b-PLA (EZLA)
As the first step, the NHS derivative of PLA was prepared. 1 g of carboxyl-terminal PLA (0.2 mmol carboxyl groups) was dissolved in 25 mL of anhydrous DMF, and placed in a flask equipped with a magnetic stirrer bar. The flask was cooled in an ice-water bath, then DCC (197.6 mg, 1 mmol) and NHS (127 mg, 1 mmol) were added. Therefore, the molar ratio of NHS and DCC to carboxyl groups on PLA was about 5:1. The reaction mixture was sealed under argon and was stirred at 0 °C for 2 h and at room temperature for 24 h. After the reaction, EZL (0.01 mmol) was dissolved in 30 mL of anhydrous DMF and injected dropwise into the solution. The detailed feeding amount of material for EZLA synthesis is presented in Table 2. The mixture was then stirred at room temperature for 24 h to allow the conjugation between the amine and carboxyl group. The crude copolymer was recovered by the rotary evaporation of solvent under reduced pressure and collected by precipitation into anhydrous diethyl ether (3 × 30 mL). After that, the polymer was dissolved in CHCl3 and the unreacted EZL and PLA remained dissolved in the mixture solution. After filtration, the laurel-green triblock copolymer EZLA was obtained and dried under vacuum at room temperature. All the purified yields were 70.2–75.9%.
Synthesis of MPEG-b-PLL-b-PLA (ELA)
A 100 mL round-bottom flask was charged with EZLA (200 mg) and TFA (8 mL). The flask was placed in an ice bath and purged under argon for 15 mins, then allowed to stir for 15 mins to ensure polymer dissolution and efficient cooling to 0 °C. After 33% HBr in HOAc (5 equivalents) was added via syringe to the polymer under argon to form a slurry and the solution was then allowed to stir in the ice bath for 1 h. The detailed feeding amount of material for EZLA synthesis is presented in Table 3. After this time, the solution was added dropwise into 20 mL diethyl ether in order to precipitate the product. The mixture was centrifuged to isolate the solid precipitate, and the product was subsequently washed with diethyl ether (20 mL) several times and followed by dialysis against distilled, deionized water (3 days) using a dialysis bag with 7.0 kDa molecular weight cut off (MWCO), then freeze-dried to yield a white solid. The white solid was dried under vacuum at 40 °C for 24 h to obtain the purified product ELA with free amino groups in the side chains. All the purified yields were 82.4–85.3%.
Preparation of micelles from EZLA and ELA
The triblock copolymer (10 mg) of EZLA or ELA was first dissolved in 10 ml of DMF to obtain a homogeneous solution, and then the polymer solution was slowly added to 10 ml of distilled water at a rate of 1 drop every 10 s with vigorous stirring. The solution was transferred into a dialysis bag (cut-off MWCO: 8.0 kDa) and dialyzed against distilled water for 1 week at room temperature to tardily remove DMF and immediately lyophilized for 2 days to obtain the micelles.
Cytotoxicity of EZLA and ELA nanoparticles
The cytotoxicity of the EZLA or ELA nanoparticles was evaluated by a cell viability assay on the Bel7402 cell line. At first, Bel7402 cells were seeded in 96-well plates at an appropriate density of cells per well in RPMI-1640 medium supplemented with 10% fetal calf serum and 1% double antioxidant. When the cells adhered well and were incubated for 24 h at 37 °C, the medium was removed and cells washed with phosphate-buffered saline (PBS). Then, the samples of EZLA or ELA nanoparticles conjugates of different concentrations in four wells with 100 μL per well: 0.032, 0.16, 0.8, 4, 20 and 100 μg/mL (5 diluted concentrations), were incubated with cells for 24 h or 72 h. Then, the medium was removed and cells washed with PBS and MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) (40 μL, 2.5 mg/mL) for 4 h. After, the MTT was removed carefully and 150 μL DMSO was added per well before shaking on the vortex turbulence apparatus for 10 mins to completely dissolve the dark blue formazan crystals inside the living cells, which was recorded on the microplate reader with absorbance measured at 570 nm. Cells relative activity equal to the average absorbance of the sample divided by the average absorbance of control group. Finally we made charts using sample concentration (μg/mL) as the abscissa and relative activity of cells (%) as the vertical axis.
Results and discussion
Design and synthesis of ELA
As described in the introduction, we aimed to synthesize amphiphilic triblock copolymers ELA. Although, Healy et al. [14]., Deng et al. [38]., Peng et al. [39]. and Lu et al. [40]. have previously reported the similar polymers, we wanted to try another general procedure to obtain amine-terminated MPEG (MPEG-NH2) from hydroxyl-terminated MPEG (MPEG-OH). The synthesis involves the conversion of the hydroxyl groups into bromo, chloro, sulfonic, and aldehyde groups, followed by other chemical reactions leading to the formation of amino groups [51, 52]. For example, Buckmann et al. [53]. and Johansson et al. [54]. reduced the brominated PEO into the corresponding alcohol using ammonia and hexadiamine, respectively. Mutter transformed a PEO end-capped with sulfonic acid groups into the corresponding amine via a classical Gabriel synthesis [55, 56]. Harris et al. [57].converted an aldehyde-PEO into the analogous amine-PEO using NaCNBH3. Besides the above strategies, Zalipsky et al. [58]. carried out an approach similar to that for the preparation of amine-terminated PCL by treating the chloro- precursor with NaN3 and Pd/C-catalyzed hydrogenation in succession. In this work, we first converted an hydroxyl-terminated MPEG into the p-toluenesulfonic acid analogue and subsequently used NaN3 [59, 60] and triphenylphosphine-catalyzed hydrogenation successively, to finally obtain the required amine-terminated MPEG. Our design was to create biodegradable backbone poly(amino acid) derivatives (EZL) by the ring-opening polymerization (ROP) of Lys(Z)-NCA with MPEG-NH2 as a macroinitiator. After which, the desired triblock copolymer ELA was synthesized by acylation of EZL using PLA-COOH and then subsequently removing the side chain protecting groups with 33% HBr/AcOH [61] to liberate the amino groups. The copolymer ELA was synthesized in three steps as shown in Scheme 1.
The synthesis of diblock copolymer EZL is a typical amine-initiated anionic polymerization. It is well known that poly(R-amino acid) can be prepared by ROP of NCA through a nucleophilic addition to the C-5 carbonyl group of the NCA with a nucleophilic initiator such as alkali, alcohols, amines, transition metals, and even water [49, 62]. Therefore, in the present study, the diblock copolymer of EZL was synthesized via the ROP of Lys(Z)-NCA using primary amine MPEG-NH2 as a macroinitiator in CHCl3. MPEG-NH2 is a kind of neutral nucleophile, and its N-terminal amino group has an unshared electron pair. Therefore, during the course of initiation and propagation an amphoteric ion of charge separation is formed, so the activity of the initiator is relatively weak. Lys(Z)-NCA is a very active monomer and can very easily form the block copolymer via the ROP, even if water is used as a macroinitiator. As a result, Lys(Z)-NCA can easily form a block copolymer with MPEG-NH2 because of its stronger activity of initiator compared with water. Because initiations are usually faster than chain propagations, the reaction immediately take places and gives off CO2 when adding the initiators, and the reaction is terminated until the monomer is exhausted. In addition, the reaction is extremely sensitive to water and O2, and so it should proceed under the protection of N2. By controlling the various feed ratios of Lys(Z)-NCA/MPEG-NH2, the diblock polymers with different contents and molecular weights can be obtained.
Finally, the acylation reaction was employed to form EZLA by coupling NHS-activated PLA-COOH to the backbone of EZL. The reaction process is relatively simple and the acylation coupling yield is more than 70%. In order to prove the success of this reaction, the molecular weights and molecular weight distributions of the as-synthesized EZLA 1-6 was measured by GPC and 1H NMR. Fortunately, the average molecular weight of EZLA is very close to that determined by 1H NMR, and the results are exhibited in Table 2. However, in order to gain the primary amine on the lysine side chain, the copolymer EZLA was then treated with a 33% solution of HBr in HAc to remove the benzyloxycarbonyl protective group in the side chain by acidolysis [63] and yield ELA with free amino group. During the deprotection reaction, trifluoroacetic acid was first added to dissolve the copolymer of EZLA, which was cooled in an ice bath. Otherwise the chain of EZLA would be easily degraded if hydrogen bromide was directly added into it. The deprotection reaction could be finished within 1 h. The resulting crude product, which was washed by ether several times, was a yellow viscous solid because of a large amount of acid present. Dialysis was carried out against deionized water (3 days) using a dialysis bag (MWCO = 7.0 kDa) to remove the excess acids and then freeze-dried to yield a white solid. In addition, the speed of adding HBr is slow enough that the heat generated form the reaction can disperse thoroughly, so the temperature of the reaction system would not rise and the carbon skeleton of the copolymer would not be broken. The amino group in the side chain of ELA can help to improve the affinity of the polymer for DNA, proteins and cells or to combine the polymer with drugs, antibodies, and DNA covalently or ionically.
Characterization of ELA
Figure 1 shows the FT-IR spectra of the block copolymers (200:1). For MPEG-NH2 (Fig. 1a), the peak at 2868 cm−1 (C-H stretch vibration) and 1,109 cm−1 (C-O stretch vibration) are characteristic peaks of the PEG block. PEG blocks were also present in the diblock prepolymer EZL (Fig. 1b), the graft triblock copolymer EZLA (Fig. 1c) and the final deprotected triblock copolymer ELA (Fig. 1d). With respect to the IR spectra for EZL (Fig. 1b), a strong peak at 3,340 cm−1 was assigned to N-H/O-H stretch vibration, the peaks at 3,065 cm−1, 1,269 cm−1, 741 cm−1 and 698 cm−1 from the phenyl group and the peak at 1,693 cm−1 from the ester carbonyl group are characteristic of PZLL block carrying benzyloxy protecting groups. Obviously, these peaks from the phenyl group and ester carboxyl group are also present in EZLA (Fig. 1c). For EZL (Fig. 1b), the absorptions at 1,641 cm−1 (amide I) and 1,539 cm−1 (amide II) are attributed to the amide group, suggesting the formation of the poly(amino acid) block. Regarding triblock copolymer EZLA (Fig. 1c) and ELA (Fig. 1d), similarly the IR spectra also shows the characteristic absorptions at 1,641 cm−1 (amide I) and 1,539 cm−1 (amide II), indicating the presence of the poly(amino acid) block (PLL). The peak at 2,944 cm−1 (C-H stretch vibration) and 1,385 cm−1 (C-H stretch vibration) are characteristics of the PLA block. PLA blocks are also present in the triblock copolymers EZLA (Fig. 1c) and ELA (Fig. 1d), which is evidence that the coupling reaction between EZL and PLA-COOH took place. The IR spectrum for ELA (Fig. 1d), in comparison to EZL (Fig. 1b) and EZLA (Fig. 1c), shows no peaks at 3,065 cm−1, 1,269 cm−1, 741 cm−1 and 698 cm−1, which are attributed to the phenyl group of the benzyloxy protecting group. Also, there is an absence of the characteristic ester peak around 1,693 cm−1 However, the N-H stretch vibration at 3,278 cm−1 indicates that the debenzylation reaction took place successfully. These FT-IR results are consistent with the expected structure of copolymer ELA.
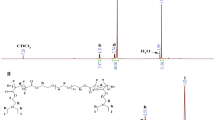
The structure of the block copolymers (200:1) of EZL, EZLA and ELA were also confirmed by 1H NMR spectra (Fig. 2). The peak d at 3.51 ppm (-CH2CH2-) is attributed to the protons of the PEG block (Fig. 2a). The characteristic peak of the PEG block can also be observed in Fig. 2b, c and d, indicating the existence of a PEG block in EZL as well as in the copolymer EZLA and ELA. With respect to EZL (Fig. 2b), the peak c at 4.21 ppm, the peak f at 2.96 ppm, and the peaks e at 1.39–1.95 ppm are assigned to (-CH-), (ε-CH2) and (β-CH2, γ-CH2 and δ-CH2), respectively Furthermore, the peaks j at 7.30–7.40 ppm (-C6H5) and the peak i at 5.02 ppm (-CH2Ph) are attributed to the phenyl group of the protecting benzyloxy group. These characteristic peaks are observed in Fig. 2c and are characteristic of the PZLL block. This demonstrates the efficient reaction of MPEG-NH2 with Lys(Z)-NCA and the incorporation of the PZLL block into the polymer backbone. The peaks a at 1.58 ppm and b at 5.17 ppm are attributed to the protons (-CH3) and (-CH-), respectively, and are assigned to the PLA block. These characteristic absorptions of the PLA block are observed in Fig. 2c and d, demonstrating successful amidation of PLA-COOH with EZL and subsequent incorporation of the PLA blocks in the copolymers EZLA and ELA. In the case of triblock copolymer ELA (Fig. 2d), the peaks at 7.30–7.40 ppm and 5.02 ppm, attributed to the phenyl group, are absent compared to the 1H NMR spectra of EZL and EZLA, demonstrating successful deprotection using 33% HBr / AcOH. The 1H NMR results are consistent with those described above obtained from the FT-IR measurements and demonstrates the successful synthesis of diblock copolymer EZL, the triblock copolymer EZLA and ELA.
The degree of polymerization of PZLL (DPPZLL) in the diblock copolymer EZL (Fig. 2b) was obtained from the integral ratio of -CH2CH2- (3.51 ppm, d) to ε-CH2 (2.96 ppm, f), as shown in the following formula, DPPZLL = (5000/44) × 2A(f)/A(d), M n(PZLL) = 262 × DPPZLL and M n(EZL) = M n(PZLL) + M n(MPEG) = M n(PZLL) + 5000. The detailed feed composition and molecular characteristics of diblock copolymer EZL are summarized in Table 1. In the same way, from the 1H NMR spectra of EZLA (Fig. 2c) and ELA (Fig. 2d), the degree of polymerization of PZLL (DPPZLL) or PLL (DPPLL) in the triblock copolymer was obtained from the integral ratio of -CH2CH2- (3.51 ppm, d) to ε-CH2 (2.96 ppm, f), as shown in the following formula DPPLL or DPPZLL = (5000)/( 44) × 2A(f)/ A(d). Thus M n( PZLL) = 262 × DPPZLL and M n( PLL) = 128× DPPLL. The chain lengths of PZLL and PLL or the molecular weights of EZLA and ELA were calculated using the known molecular weights of PEG and PLA. The detailed compositions and the molecular weights of EZLA and ELA are summarized in Tables 2 and 3.
The molecular weights and molecular weight distributions of the final deprotected triblock copolymers ELA1 to ELA6 were also confirmed by GPC as illustrated in Fig. 3a, b, c, d, e and f. After purification, the unimodal, symmetrical and homogeneous molecular weight distributions in the GPC traces indicated single component and no or negligible homopolymer residue in these polymers. The GPC results indicate that the polymer chain backbone was not broken by treatment with HBr in HOAc and the deprotection reaction took place without any unwanted side reactions. The GPC peaks of the resulting copolymers (ELA) gradually shifted to higher molecular weights by increasing the feed ratios of the monomer and initiator. The chain lengths of each block can be controlled by varying the feed ratios of Lys(Z)-NCA and the starting material (MPEG-NH2), in other words by changing the monomer/initiator ratios in the ring-opening polymerization. Almost quantitatively molecular weights were achieved and the molecular weights and molecular weight distributions of these polymers are analyzed in Table 3. The number average molecular weights (from 21,600 to 36,300) of these copolymers slightly increase when the monomer/initiator ratios are increased. However, the values of M w/M n are not significantly changed and the polymers exhibit a narrow molecular weight distribution (M w /M n = 1.10–1.25). The molecular weights and molecular weight distributions of these polymers are in good agreement to those calculated from the 1H NMR spectra. The narrow molecular weight distributions of these polymers shows that the reactivity of each deprotection reaction was high enough and the final product did not contain unreacted EZLA. The GPC results were performed to verify the successful synthesis of these final triblock copolymers ELA.
The second heating thermograms of the EZL diblock and EZLA tiblock copolymers by differential scanning calorimetry (DSC) are shown in Fig. 4. For EZL (Fig. 4a) the melting peaks at 58.9 °C and 274.9 °C are attributed to the MPEG blocks and PZLL blocks, respectively. The characteristic peaks of the MPEG (57.6 °C) and PZLL (273.5 °C) blocks are also observed in Fig. 4b for the copolymer EZLA. In the DSC scan for EZLA there is an additional melting peak at 158.9 °C (Fig. 4b), compared to the DSC scan for EZL, which is assigned to the PLA block. This demonstrates the successful synthesis of the tirblock copolymer EZLA. The T m of EZL and EZLA are summarized in Table 4.
Both the morphology and the average size of the copolymer nanoparticles of EZLA or ELA were investigated by the techniques of TEM and DLS, and the detail results were as shown in Figs. 5 and 6. First polymeric micelles of the copolymers EZLA or ELA were formed in aqueous solution, dropped in a copper mesh and were subsequently negatively stained with phosphotungstic acid (0.5 wt %) and finally dried at room temperature. From DLS (Fig. 5), the polymeric micelles formed from EZLA showed mono size distribution with PDI 0.42 and the mean size was determined to be 58.8 nm (Fig. 5a), and that of the polymeric micelles formed from deprotected ELA were 0.11 and 68.1 nm, respectively (Fig. 5b). From TEM (Fig. 6), it was found that the polymeric micelles were all with good size uniformity and good dispersion. The polymeric micelles formed from EZLA were adhesive spherical in shape and the particle size of EZLA was about 40–50 nm (Fig. 6a), while the ELA was self-assembled into isolated spherical micelles and the particle size of ELA was about 50–60 nm (Fig. 6b). The results were in good agreement with that of the DLS observations. Figure 7 shows the self-assembly schematic diagram of the triblock copolymers EZLA and ELA. It was found that the EZLA micelles had a hydrophobic PLA-b-PZLL block core surrounded by a hydrophilic MPEG shell and the ELA micelles had a hydrophobic PLA core surrounded by a hydrophilic MPEG-b-PLL block shell. The adhesive spheric micelles which look like worms in Fig. 6a can be attributed to the fact that the hydrophobic PLA and PZLL blocks intertwined each other to some extent, resulting in deformation of spherical micelles. And the deprotected copolymer ELA can self-assemble into nearly spherical micelles and the average diameter of them was bigger than that of EZLA, which maybe due to the electrostatic repulsion of the amino groups of PLL after deprotection.
The cell-cytotoxicity of the ELA and EZLA polymeric micelles were evaluated by the widely established MTT assay performed with Bel7402 human hepatoma cells, as shown in Fig. 8. Samples of EZLA and ELA nanoparticle conjugates were 5-fold diluted in concentration from 100 μg/mL to 0.032 μg/mL, and incubated with cells for 24 h or 72 h. Figure 8 shows that cytotoxicities of ELA and EZLA are dose- and time-dependent. Although the cell activity decreases when the concentration of the sample increases, the degree of apoptosis caused by ELA was very low, and even at high concentration of 100 μg/mL the cell death rate was only about 20%. In addition, as time increases the cell activity affected by the samples of the ELA becomes weakened, however, this effect is not obvious. However, the cell death rate of EZLA was relatively on the high side under the same experimental condition. But even at high concentration of 20 μg/mL the cell death rate was no more than 20%. Generally speaking, drug concentration is about 10 μg/mL in actual applications, so they are both in the reasonable scope of biological applications. This means that ELA and EZLA have good biocompatibility if used in biological research. Moreover, the similarity of the cytotoxicity of ELA and EZLA indicates that the presence or absence of the protecting benzyloxy group, which is an important structural feature of ELA and EZLA, have negligible effect on cell metabolism even though the deprotected polylysine was charge-positive in aqueous solution. Studies of the mechanical properties of selected stereo complexes and studies of their usefulness in drug release devices are in progress and will be reported later.
Conclusions
In a few straightforward steps, the degradable ABC-type amphiphilic triblock copolymers, MPEG-b-PLL-b-PLA (ELA), consisting of hydrophilic segments (PLL and MPEG) and hydrophobic PLA segments were successfully synthesized by acidolysis of MPEG-b-PZLL-b-PLA (EZLA) to remove the side chain protecting groups with HBr. While starting from PEG, the triblock copolymers MPEG-b-PZLL-b-PLA (EZLA) were obtained by amidation of PLA-COOH with MPEG-b-PZLL-NH2 (EZL). The latter was synthesized from the ring-opening polymerization (ROP) of Lys(Z)-NCA with amino-terminated PEG (MPEG-NH2) as the macroinitiator. The chemical structures of the intermediate and final products were confirmed by 1H NMR, FT-IR, GPC and DSC. The results confirmed that the chain length of each block polymer could be controlled by adjusting the feed ratios of the monomer Lys(Z)-NCA and MPEG-NH2. The TEM results demonstrate that the copolymers EZLA and ELA easily form polymeric micelles in aqueous solution and the polymeric micelles are spherical in shape. Moreover, the obtained polymeric micelles exhibit no obvious cell-cytotoxicity against Bel7402 human hepatoma cells and should be useful for biodegradable biomedical materials such as drug and gene delivery vehicles.
References
Heiger-Bernays WJ, Essigmann JM, Lippard SJ (1990) Biochemistry 29(36):8461–8466
Gottesman MM, Fojo T, Bates TSE (2002) Nat Rev Cancer 2:48–58
Garrec DL, Gori S, Luo L, Lessard D et al (2004) Journal of Controlled Release 99(1):83–101
Urry DWJ (1988) Protein Chem 7:81–114
Cappello J, Crissman JW (1990) Polym Prepr 31:193–194
Waite JH (1990) Polym Prepr 31:181–182
Matsusaki M, Waku T, Kaneko T, Kida T (2006) Akashi M Langmuir 22:1396–1399
Chécot F, Lecommandoux S, Gnanou Y, Klok HA (2002) Angew Chem Int Ed 41:1339–1343
Holowka EP, Pochan DJ, Deming TJJ (2005) Am Chem Soc 127:12423–12428
Wong MS, Cha JN, Choi KS, Deming TJ, Stucky GD (2002) Nano Lett 2:583–587
Varga CM, Tedford NC, Thomas M, Klibanov AM, Griffith LG, Lauffenburger DA (2005) Gene Ther 12:1023–1032
Douglas KL (2008) Biotechnol Prog 24:871–883
Park TG, Jeong JH, Kim SW (2006) Adv Drug Delivery Rev 58:467–486
Park S, Healy KE (2003) Bioconjugate Chem 14:311–319
Makiya N, Leaf H (2001) Human Gene Therapy 12(8):861–870
Jiang GH, Wang L, Yu HJ, Chen C, Dong XC, Chen T et al (2006) Polymer 47:12–17
Jiang GH, Wang L, Chen T, Yu HJ, Dong XC, Chen C (2005) Polymer 46:9501–9507
Yu X, Tang XZ, Pan CY (2005) Polymer 46:11149–11156
Signori F, Chiellini F, Solaro R (2005) Polymer 46:9642–9652
Yuan M, Wang Y, Li X, Xiong C, Deng X (2000) Macromolecules 33:1613–1617
Deng M, Wang R, Rong G, Sun J, Zhang X, Chen X, Jing X (2004) Biomaterials 25:3553–3558
Kricheldorf HR, Kreiser-Saunders I, Juergens C, Wolter D (1996) Macromol Symp 103:85–102
Eling S, Gogolewski B, Pennings JA (1982) Polymer 23:1587–1593
Chabot F, Vert M, Chapelle S, Granger P (1983) Polymer 24:53–59
Jeoung YS, Kim WS (1986) Arch Pharm Res 9:63–73
Langer R (2000) Acc Chem Res 33:94–101
Agrawal CM, Athanasiou KA, Heckman JD (1997) Mater Sci Forum 250:115–128
Hench LL (1998) Biomaterials 19:1419–1423
Han DK, Hubbell JA (1996) Macromolecules 29:5233–5235
Zeng J, Xu XY, Chen XS, Liang QZ, Bian XC, Yang LX et al (2003) J Controlled Release 92:227–231
Ertl B, Platzer P, Wirth M, Gabor FJ (1999) Controlled Release 61:305–317
Albertsson AC, Varma IK (2003) Biomacromolecules 4:1466–1486
Wang Q, Wang YJ (2011) Polym Res 18:385–391
Wang T, Jiang M, Wu YJ (2010) Polym Res 17:335–345
Xiong, Jiang HW, Wang DZ (2009) J Polym Res 16:191–197
Yokoyama M, Inoue S, Kataoka K, Yui N, Okano T, Sakurai Y (1989) Makromol Chem 190:2041–2054
Harada A, Kataoka K (1995) Macromolecules 28:5294–5299
Deng C, Chen XS, Yu HJ, Sun J, Lu TC, Jing XB (2007) Polymer 48:139–149
Peng H, Xiao Y, Mao XL, Chen L, Crawfordand R, Whittaker AK (2009) Biomacromolecules 10:95–104
Lu TC, Sun J, Chen XX, Zhang PB, Jing XB (2009) Macromol Biosci 9:1059–1068
Xiang L, Shen LJ, Long F, Yang K, Fan JB, Li YJ, Xiang JN, Zhu MQ (2011) Macromol Chem Phys 212:563–573
Harada A, Cammas S, Kataoka K (1996) Macromolecules 29:6183–6188
Cho C, Cheon J, Jeong Y, Kim I, Kim S, Akaike T (1997) Macromol Rapid Commun 1:361–369
Lavasanifar A, Samuel J, Kwon G (2002) Adv Drug Delivery Rev 54:169–190
Tang DM, Lin JP, Lin SL, Zhang SN, Chen T, Tian XH (2004) Macromol Rapid Commun 25:1241–1246
Li T, Lin JP, Chen T, Zhang SN (2006) Polymer 47:4485–4489
Sun J, Chen XS, Deng C, Yu HJ, Xie ZG, Jing XB (2007) Langmuir 23:8308–8315
Daly WH, Poché D (1988) Tetrahedron Lett 29:5859–5862
Dormanl C, Shiang WR, Meyers PA (1992) Synth Commun 22:3257–3262
Perrin DD, Armarego WLF, Perrin DR (1980) Purification of Laboratory Chemicals. Pergamon Press, Oxford U.K
Wang W, Li L, Helm G, Zhou H, Li AJ (2003) Am Chem Soc 125:1120–1121
Lu FZ, Xiong XY, Li ZC, Du FS, Zhang BY, Li FM (2002) Bioconjugate Chem 13:1159–1162
Buckmann AF, Morr M, Johansson G (1981) Makromol Chem 182:1379–1384
Johansson G, Gysoin R, Flanagan SDJ (1981) Biol Chem 256:9126–9135
Mutter M (1978) Tetrahedron Lett 31:2839–2842
Ciuffarin E, Isola M, Leoni PJ (1981) Org Chem 46:3064–3070
Harris JM, Yalpani M, Van AJM, Struck EC, Case MG, Parley MS, Brooks DE (1984) J Polym Sci Polym Chem Ed 22:341–352
Zalipsky S, Gilon C, Zilkha A (1983) Eur Polym J 19:1177–1183
Garanti L, Molteni G (2003) Tetrahedron Lett 44:1133–1135
Hiki S, Kataoka K (2007) Bioconjugate Chem 18:2191–2196
Benishia D, Berger AJ (1952) Org Chem 17:1564–1570
Blout ER, Karison RHJ (1956) Am Chem Soc 78:941–950
Pytela J, Jakes J, Rypacek F (1994) Int J Biol Macromol 16:15–20
Acknowledgements
The authors greatly acknowledge the financial support from the National Natural Science Foundation of the People’s Republic of China (20874025), the Fundamental Research Funds for the Central Universities (HUST2010MS101), Department of Science and Technology Foundation of Hunan Province (2009WK4005), the Program for New Century Excellent Talents in Universities (NCET-07-00273) and the “973” National Key Basic Research Program of China (2007CB310500).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, MQ., Xiang, L., Yang, K. et al. Synthesis and characterization of biodegradable amphiphilic triblock copolymers methoxy-poly(ethylene glycol)-b-poly(L-lysine)-b-poly(L-lactic acid). J Polym Res 19, 9808 (2012). https://doi.org/10.1007/s10965-011-9808-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-011-9808-y