Abstract
Effects of poly(ethylene glycol) (PEG) block length on the self-assembly of a series of pH-sensitive poly(N,N-diethylaminoethyl methacrylate)m-PEGn-poly(N,N-diethylaminoethyl methacrylate)m triblock copolymers (PDEAEMAm-PEGn-PDEAEMAm) have been studied. The synthesized triblock copolymers have an almost fixed PDEAEMA block length (m ≈ 65) and varying PEG block lengths (n = 11, 20, 89 or 134). The acid–base titration results show that the pKa value of the block copolymer shifts from 6.84 to 7.08 when the PEG block length increases from 11 to 134, indicating that the pH-sensitivity of the triblock copolymer can be adjusted through changing the PEG block length. With the increase of the hydrophilic PEG block length, the critical micelle concentration value of the triblock copolymer obtained from fluorescence spectroscopy changes from 5.50 × 10–3 to 13.23 × 10–3 mg mL−1. The block copolymers can self-assemble into the micelles in PBS solution at pH 7.4, and the average size of the self-assembly decreases from 178 nm to 45 nm with the increase of the PEG block length as determined by dynamic light scattering. The block copolymers show great biocompatibility in the cytotoxicity assay. Results from these triblock copolymers in the drug-controlled release indicate that the release rate of doxorubicin-loaded micelles at pH 5.0 is faster than that at pH 7.4, and the cumulative drug release in 48 h at pH 5.0 increases from 70.4% to 89.8% with the decrease of the PEG block length. Accordingly, this PDEAEMA65-PEGn-PDEAEMA65 copolymer is an excellent carrier for controllable drug delivery and release.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Block copolymer based nano-carriers serving as drug delivery systems have attracted tremendous interests recently due to the potential applications in the cancer therapy etc. [1,2,3]. The biocompatibility and biodegradability must be considered when the self-assembly of block copolymers are used for the drug delivery devices. Poly(ethylene glycol) (PEG) possesses lots of outstanding physicochemical and biological properties such as hydrophilicity, solubility in water and different organic solvents, and lack of toxicity [4,5,6]. Thus, PEG has been widely applied as a hydrophilic segment in developing block copolymeric nanoparticle systems [7,8,9]. Furthermore, some block copolymers can rapidly change their conformations and aggregations by changing the external environments such as pH [10,11,12], temperature [13, 14], ionic strength [15], or solvents [16, 17]. These stimuli responsive copolymers play an important role in the drug delivery systems [18, 19]. Indeed, pH-sensitive micelles are more attractive [20], since defined pH gradients exist in endosomes (pH 6.5–5.0), lysosomes (pH 5.0–4.5), extracellular medium of normal tissue and blood (pH 7.4), and the more acidic environment in cancer cells compared to healthy cells. Thus, the effects of pH media on the self-assembly of block copolymers have been considerably investigated. For example, Oh and co-workers [21] designed a pH-triggered surface charge-switched micelles of poly(L-lactic acid)-PEG-poly(L-lysine-Nε-(2,3-dimethyl maleic acid)). When the pH was lower than 6.5, the micelles disintegrated and released drugs inside the core. Poly(N,N-diethylaminoethyl methacrylate) (PDEAEMA) is a cationic polymer with tertiary amine groups, and has been proved to be an efficient pH sensitive polymer with good biocompatibility and a pKa value of about 7.3 [22,23,24,25,26]. Many researches have been focused recently on block copolymers containing PDEAEMA blocks such as poly(ε-caprolactone) (PCL)-PDEAEMA [27,28,29], PEG-PDEAEMA [30,31,32], PEG-PCL-PDEAEMA [33], which are pH sensitive and can be used for anti-cancer drug carries.
Besides external environments, the self-assembly of block copolymers is also affected greatly by chemical compositions, block architectures, block lengths, and pendent structures [17, 34,35,36,37,38,39]. For example, Dong and co-workers synthesized a series of PEG-poly(glycidyl methacrylate) (PEG-PGMA) diblock copolymers by the method of atom transfer radical polymerization (ATRP), and the diblock copolymers were modified by aldehyde to obtain pH sensitivity. It was found that the longer hydrophobic PGMA block resulted in a higher doxorubicin (DOX) loading but a bigger particle size in the self-assembled micelles [40]. Verkoyen and co-workers synthesized a series of poly(long-chain alkyl glycidyl ethers)-PEG-poly(long-chain alkyl glycidyl ethers) triblock copolymers (PAlkGE-PEG-PAlkGE) through anionic ring opening polymerization, and the results showed that due to self-assembly of the hydrophobic alkyl chains, the addition of water to the triblock copolymers led to the formation of micelles. By varying the hydrophobic PAlkGE block length, a tunable hydrophobic effect can be achieved [41].
Length and content of the PEG chain play important roles in the self-assembly and properties of PEG based copolymers [42, 43]. For example, compared with the short PEG block length, PEG-b-poly(dimethylaminoethyl methacrylate-co-propylacrylic acid-co-butyl methacrylate) (PEG-b-pDPB) with long PEG block length had a larger micelle size and a lower cellular uptake [44]. Polyethylenimine (lPEI)-g-PEG micellar nanoparticles prepared with short PEG grafts showed comparable colloidal stability in salt and serum-containing media, and displayed significantly higher in vitro transfection efficiency compared to those prepared with longer PEG grafts [6]. In our previous study, we investigated how the degrees of polymerization (DP) of PDEAEMA affects the pH-responsive, self-assembly and drug delivery of the PDEAEMAm-PEG43-PDEAEMAm triblock copolymers. Here, we synthesized a series of PDEAEMA65-PEGn-PDEAEMA65 triblock copolymers through the method of ATRP using Br-PEGn-Br macroinitiators with different DP values. Then, the influence of the hydrophilic PEG block length on the self-assembly behavior, pH-sensitivity, critical micelle concentration (CMC) of the triblock copolymers was investigated with the aid of fluorescence spectrum, UV–visible spectrophotometer (UV–vis), transmission electron microscopy (TEM), and dynamic light scattering (DLS) spectrophotometer. The drug-loading content, and the drug release behavior of DOX were investigated. Furthermore, in vitro cytotoxicity of the triblock copolymer micelles was also studied.
Experimental
Materials
PEG with number-average molecular weight (Mn) of 600, 1000, 4000 and 6000 Da, namely PEG-600, PEG-1000, PEG-4000 and PEG-6000, respectively, was purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. All PEG samples were dried at 105 °C under vacuum to remove residual water prior to use. 2-bromoisobutyl bromide (BIBB) was purchased from TCI. N,N-diethylaminoethyl methacrylate (DEAEMA) and triethylamine (TEA) were supplied by Aldrich. Copper(I) bromide (CuBr), tetrahydrofuran (THF), dichloromethane, N,N,N′,N′,N′′-pentamethyldiethylenetriamine (PMDETA), and methanol were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Doxorubicin hydrochloride (DOX•HCl, 99%) was provided by Yuancheng Create Technology Co., Ltd., China. DOX, DEAEMA, TEA, THF, CuBr, methanol, and PMDETA were purified according to the procedures in the reference [12]. All other materials were used as received.
Synthesis of Br-PEGn-Br macroinitiator
α,ω-Dibromo PEG macroinitiators (Br-PEGn-Br) with different DP values of 11, 20, 89, and 134, respectively, were synthesized according to previously reported process [12]. Typically, PEG with different Mn (10 mmol), TEA (4.05 g, 40 mmol), and dry dichloromethane (150 mL) were placed into a dry and argon purged flask equipped with an addition funnel. Then the system was kept in an ice bath. BIBB (9.2 g, 40 mmol) diluted with 60 mL dry dichloromethane was added dropwise to the flask while stirring. After stirred for 24 h at ambient temperature, the solution was filtered to remove salts. The solution was concentrated and then precipitated in ethyl acetate. The crude precipitate was dissolved in dichloromethane, extracted successively with 0.1 M HCl and saturated sodium bicarbonate solution three times to remove salts and TEA. The isolated solution was dried with anhydrous magnesium sulfate, and the solvent was removed under vacuum after filtration. The Br-PEGn-Br was gotten after drying under vacuum to a constant weight.
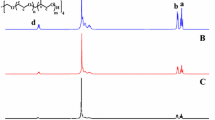
1H NMR (400 MHz, CDCl3, RT, ppm) (Br-PEG11-Br, Fig. 1a): 1.93 (s, (CH3)2C-Br); 3.63 (s, -OCH2CH2O-); 3.74 (s, OCH2CH2OC(= O)); 4.31 (s, OCH2CH2OC(= O)).
Preparation and characterizations of PDEAEMA65-PEGn-PDEAEMA65 triblock copolymers
Typically, Br-PEGn-Br (0.11 mmol), DEAEMA 3.67 g (19.8 mmol), CuBr 31.9 mg (0.22 mmol), and 5.5 mL methanol were added in a Schlenk flask equipped with a magnetic stirrer, and the flask was evacuated and flushed with argon twice before use. After three freeze–pump–thaw cycles, PMDETA 38.1 mg (0.22 mmol) was added under argon atmosphere. The mixture was heated at 25 ℃ for 2 h with stirring under argon atmosphere. The reaction was stopped with liquid nitrogen, then the crude product was diluted with THF and passed through an alumina column to remove the catalysts. After the concentrated process under vacuum, the resulting solution was precipitated in cold n-hexane. The final product was obtained after dried at 40 ℃ for 24 h under vacuum. The synthetic routes are shown in Scheme 1.
Molecular weights and molecular weight distributions were measured by a conventional gel permeation chromatography (GPC) system equipped with a Waters 1515 isocratic HPLC pump, a Waters 2414 refractive index detector. GPC measurements were carried out at 40 ℃ in THF with a flow rate of 1.0 mL min−1. The system was calibrated with linear polystyrene standards. 1H NMR data were obtained by a Bruker AVANCE 400 MHz NMR spectrometer with CDCl3 as solvent at room temperature.
1H NMR (400 MHz, CDCl3, RT, ppm) (Fig. 1b): 0.88 (s, α-CH3 of PDEAEMA); 1.02 (s, -N(CH2CH3)2); 1.79–2.06 (m, -CH2-C- of PDEAEMA and -CH3 in backbone); 2.55 (s, -N(CH2CH3)2); 2.68 (s, -NCH2CH2O-); 3.97 (s, -NCH2CH2O-).
Preparation and characterizations of micelles
Micellization of the triblock copolymers was obtained by a co-solvent process. The triblock copolymer was dissolved into THF to get a solution with a concentration of 1 mg mL−1, then phosphate buffered solution (PBS, 0.02 M, pH 7.4) was added dropwise to this THF solution under vigorous stirring until the PBS content reached 50%, then continued to stir at room temperature for 24 h. Finally, the solution was dialyzed against PBS for 48 h (molecular weight cutoff = 3500 Da) to remove THF, and the PBS was exchanged every 2 h for the first16 h and every 8 h for the next 32 h.
The transmittance of micelles with a concentration of 1 mg·mL−1 was determined with a JingHua 759S UV–vis spectrophotometer. The pH values of micelles were gradually increased from 2 to 11 and the solution was equilibrated at each pH for 24 h.
The CMC of the triblock copolymers was determined by the fluorescence probe technique using pyrene as a fluorescence probe.
Hydrodynamic diameters (Dh) and size distributions (PDI) of micelles in aqueous solutions were measured by a Malvern Zetasizer Nano-ZS DLS Instrument equipped with a 22 mW He–Ne laser (λ = 632.8 nm) and at an operating angle of 173°.
The morphology of micelles was observed by TEM. All samples were obtained at an accelerating voltage of 100 kV using a JEM 1400 (HITACHI). The samples were prepared by placing a drop of micelles on the copper grid with carbon films and dried in air at room temperature before measurements.
Acid–base titration was designed to evaluate the buffering capacity of micelles, which were recorded on a pH meter equipped with a LE438 composite electrode. The specific progress was as follows: the pH value of micelles with a concentration of 1 mg mL−1 was adjusted to 2.00 via the addition of HCl aqueous solution (1.0 M). The increase of the pH values of micelles was recorded using the pH meter after adding 0.1 M NaOH aqueous solution.
The cytotoxicity of micelles was investigated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylte-trazoliumbromide (MTT) assay. Human lung adenocarcinoma cells (A549) or mouse fibroblast cells (L929) were seeded at a density of 5000–7000 cells per well in a 96-well plate and incubated for 24 h under a 5% CO2 atmosphere at 37 °C. Then 200 mL distilled water (8 control wells per plate) and polymeric micelles (final concentrations were 12.5, 25, 50, 100 and 200 μg mL−1) were added into the wells, and cells were further cultured for another 24 h. 20 mL MTT solution (0.5 mg mL−1) in PBS buffer (pH 7.4) was added to each well. After incubation for 4 h, the resulting formazan crystals were dissolved by 150 mL of dimethyl sulfoxide and shook for 10 min. The absorbance (OD value) at 490 nm was measured using a microplate reader. The cell viability was calculated by the Eq. (1).
where ODcontrol and ODtest are the average OD values of control group and micelles, respectively.
DOX-loaded micelles were prepared as follows [12]. 1 mg DOX and 10 mg copolymer were dissolved in 10 mL THF. This solution was added to 5 mL of PBS (0.02 M, pH 7.4) in a dropwise manner. After stirring at room temperature for 24 h, the mixture solution was transferred into a cellulose membrane bag (molecular weight cutoff = 3500 Da) and dialyzed against the PBS to remove free DOX. Subsequently, after lyophilization, the blank micelles and DOX-loaded micelles were obtained and stored at -20 ℃ for further experiments. UV–vis absorption was measured in order to calculate the drug loading content (DLC) and the drug loading efficiency (DLE) using Eqs. 2 and 3, respectively. The drug release of DOX-loaded micelles was quantified using UV–vis spectrophotometer by monitoring the absorbance at 480 nm.
A solution of DOX-loaded micelles (10 mL) was added to a cellulose membrane bag (molecular weight cutoff = 3500 Da) and dialyzed against 200 mL PBS with different pH values respectively (pH 7.4 and 5.0) at 25 ℃. The volume of dialysis fluid was ensured to be about 200 mL during the measurement. At different time intervals, the liquid outside the dialysis bag was taken out and the same volume of fresh buffer was added. The liquid was measured with the UV–vis spectrophotometer at 480 nm to obtain the cumulative release curve of DOX.
Results and discussion
Chemical structures of the macroinitiators and the triblock copolymers
α,ω-Bromopropionyl PEG (Br-PEGn-Br) with different DP values were synthesized by the reaction between BIBB and PEG with different molecular weights (Scheme 1). The attachment of the 2-bromoisobutyrl group is confirmed by the 1H NMR spectrum (Fig. 1A). A singlet appears at 1.93 ppm corresponding to the two methyl groups (peak c) adjacent to the bromine. The hydroxyl end groups are fully transformed into the corresponding 2-bromoisobutyrl moiety, as determined by the integrated area ratio (about 1:3) of peaks b and c. Furthermore, the DP value can be calculated according to the integrated area ratio of peaks (a + d) and b, and the results are listed in Table 1, which show that the DP values of PEG macroinitiators range from 11 to 134. The GPC traces (Fig. 2A) of Br-PEGn-Br exhibit unimodal and symmetrical peaks with very narrow molecular weight distributions (Mw/Mn = 1.04–1.08, Table S1), which also confirms that the PEG macroinitiators with different DP values were synthesized successfully.
In parallel with our previous study, PDEAEMA65-PEGn-PDEAEMA65 triblock copolymers were successfully synthesized (Scheme 1), the chain length of PEG block (DP = 11, 20, 89, 134) is varied and the chain length of PDEAEMA block (DP ≈ 65) is fixed. The reaction was performed in methanol at 25 ℃ using CuBr/PMDETA as the catalytic system. The chemical structures of the purified triblock copolymers are characterized by 1H NMR and GPC spectroscopies. All GPC curves (Fig. 2B) of these triblock copolymers are unimodal and symmetrical, and the detailed information about the Mn, GPC and Mw/Mn of the triblock copolymers is listed in Table 1. The triblock copolymers have narrow Mw/Mn values (< 1.38) and higher Mn, GPC values than the corresponding Br-PEGn-Br macroinitiators (Table S1), indicating the achievement of purified triblock copolymers. 1H NMR (Fig. 1B) spectral analysis shows that these copolymers are in good accordance with their expected chemical compositions. The integrated area ratio of -OCH2CH2O- in the PEG block (3.63 ppm) and –N(CH2CH3)2 in the PDEAEMA blocks (1.02 ppm) confirms that all the triblock copolymers have almost the same DP values of PDEAEMA blocks (about 65). The results indicate that the triblock copolymers with a fixed PDEAEMA block length and different PEG block lengths are prepared successfully. The Mn, GPC values of triblock copolymers are higher than those calculated by 1H NMR spectra (Mn, NMR), which is probably due to the different polarities between the triblock copolymers and polystyrene standards. In this regard, Mn, NMR values are chosen to represent the Mn of triblock copolymers.
pH-sensitivity of the triblock copolymers
pKa is an important parameter to characterize pH responsive polymers, and the apparent pKa value of micelles can be obtained by acid–base titration. Many reports showed that PDEAEMA-based copolymer micelles displayed different pKa values when the length of PDEAEMA segments was changed [12, 33]. In our present study, the triblock copolymers also exhibit different pKa values due to varying length of PEG block. The acid–base titration curves of the synthesized triblock copolymer micelles are presented in Fig. 3A, and the obtained average pKa values are listed in Table 2. Compared to pure water, the triblock copolymer micelles clearly show pH buffering platforms, which are resulted from the protonation of diethylamino (DEA) groups in the PDEAEMA blocks. When the lengths of PDEAEMA blocks are kept almost constant, the longer the PEG block, the higher the pKa values of the copolymers (Table 2), demonstrating that the pKa value of the copolymer can be adjusted by tuning the ratio of hydrophilic PEG segments, which may be attributed to the shielding effect of PEG shell. The PEG segments act as the thick shell of the micelles, which forbid the contact of DEA groups with the aqueous solution, in this way, it decreases the electrostatic repulsion and improves the pKa value.
The pH-sensitivity was further evaluated by the transmittance of the copolymer micelles (1 mg mL−1) as presented in Fig. 3B. The curves show sharp transitions from transparent solutions (100% transmittance) to turbid mixtures (0% transmittance) within pH region from pH 6.8 to 8 for PDEAEMA67-PEG11-PDEAEMA67 and PDEAEMA65-PEG20-PDEAEMA65. But the decrease of transmittance with the increase of pH value is small for PDEAEMA67-PEG89-PDEAEMA67 and PDEAEMA65-PEG134-PDEAEMA65. The latter two copolymers with longer PEG blocks can form nano-size micelles even at pH 7.4 (discussed as follows), resulting in the almost unchanged transmittance when the acidic environment becomes basic. Thus, with the increase of the PEG block length, the aqueous solutions of the triblock copolymers appear transparency at basic conditions.
Micellization properties of the triblock copolymers
The CMC values determined by fluorescence spectroscopy (Fig. S1) are given in Table 2 [12]. It is found that when the DP of the PEG block increases from 11 to 134, the CMC values increase from 5.50 × 10–3 to 13.23 × 10–3 mg mL−1. The results clearly show that all the prepared triblock copolymers can self-assemble into micelles even at an ultra-dilute concentration, indicating that the micelles would be stable during the dilution when entering blood circulation [33]. Generally, the CMC value of amphiphilic block copolymers increases with the increasing length of the hydrophilic polymer block due to the enhanced hydrophilicity of whole polymer chains [45]. In our previous study, we also found that CMC value decreased with the increase of the hydrophobic PDEAEMA block length [12].
Since PDEAEMA is a pH sensitive block, the Dh and PDI of the prepared triblock copolymer micelles at two conditions (pH = 7.4 and 6.5) were measured by DLS (Fig. S2, Table 3). The Dh values of all the micelles at pH 7.4 are less than 180 nm and the PDIs are relatively narrow, which implies that the micelles are potential for drug carriers. In addition, with the increasing DP of the hydrophilic PEG block from 11 to 134, Dh values at pH 7.4 decrease from 178 to 45 nm. This may be due to the obviously enhanced content of the hydrophilic PEG chains. With the decrease of pH value from 7.4 to 6.5, the Dh values of all the micelles decrease sharply to around 30 nm. This change is related to the hydrophobic PDEAEMA segments turned into hydrophilic through the protonation of DEA groups under the pH 6.5 condition (pKa > 6.5 seen in Table 2). Then, the electrostatic repulsion of PDEAEMA blocks becomes so strong that the copolymer molecules can no longer hold together, leading to the decreasing micelle sizes.
TEM images (Fig. 4) also reveal that the prepared triblock copolymers self-assemble into near-spherical micelles [32], and the particle sizes of the self-assembly decrease with the increase of PEG block length. However, the Dh of the micelles from TEM images (Table 3) is smaller than the value obtained from DLS measurements. This may be due to the fact that the size of micelles can be directly measured in aqueous solution via DLS, while the micelle samples used in TEM tests were dehydrated [46,47,48].
In vitro cell cytotoxicity assay
The in vitro cytotoxicity of the blank triblock copolymer micelles was evaluated by MTT assay with A549 and L929 cells. The results are shown in Fig. 5. Figure 5 shows that the cell viability is higher than 90% after 24 h incubation even for micelle concentration up to 200 μg mL−1, indicating that the as-prepared PDEAEMA65-PEGn-PDEAEMA65 micelles are nontoxic to A549 and L929 cells.
In vitro drug loading and release
In order to assess the suitability of the prepared copolymer micelles as drug delivery carriers, encapsulation of the hydrophobic DOX in the micelles and the release study of the loaded micelles are performed. The DLC and DLE values (Table 4) have a decreasing trend with the increase of the hydrophilic PEG block length. PDEAEMA67-PEG11-PDEAEMA67 and PDEAEMA65-PEG20-PDEAEMA65 have much higher DLC and DLE values compared to the other two copolymers. This may be due to the bigger hydrophobic core formed by PDEAEMA blocks which can encapsulate more hydrophobic DOX in the micelles, as the decease of the PEG block length leads to higher PDEAEMA block content.
Fig. S2b shows the DLS curves of the DOX-loaded triblock copolymer micelles. Table 3 also indicates that the Dh values of the blank micelles at pH 7.4 are significantly lower than those of the correspondent DOX-loaded micelles, which imply that the drug, DOX, is encapsulated into the triblock copolymer micelles effectively. Furthermore, the Dh values of the DOX-loaded micelles increase with the decreasing length of the hydrophilic PEG block, which indicates that more drugs are encapsulated in the shorter PEG block copolymer micelles [49].
In vitro drug release experiments are performed at pH 7.4 and pH 5.0 for the DOX-loaded micelles. The release profiles are displayed in Fig. 6 and the cumulative release amount in 48 h is summarized in Table 4. The profile of release curves under the condition of pH 7.4 is similar for all four kinds of triblock copolymer micelles. At pH 7.4, around 30% of DOX is released in 10 h and then the release rates are almost unchanged, at last, less than 34% of DOX is released in 48 h, indicating that the release rate of the drug is low at pH 7.4, resulting from the protection effect of the tight structure of micelles. At pH 5.0, the release rates of DOX accelerate significantly. About 70% of DOX is released in 10 h, then the release amount reaches about 80% in 48 h. Most of the DEA groups in PDEAEMA blocks have been protonated under pH 5.0, resulting in the swollen of the drug-loaded micelles, which should accelerate the release of DOX [29]. At pH 7.4, the cumulative drug release in 48 h is almost the same, however at pH 5.0, it increases from 70.4% to 89.8% with the decrease of the PEG block length from 134 to 11 (Table 4). This may be due to the fact that shorter PEG block would increase the content of pH-sensitive PDEAEMA blocks, and further enhance the micelle expansion with the decrease of the pH value, which is in favor of drug release from the micelles.
Conclusion
In this work, a series of well-defined biocompatible PDEAEMA65-PEGn-PDEAEMA65 triblock copolymers with a fixed PDEAEMA block length (m ≈ 65) and different PEG block lengths (n = 11, 20, 89 and 134) were successfully prepared using different Br-PEGn-Br as macroinitiators. The hydrophiles of the whole polymer increase with the increasing length of the PEG block, which has great impacts on the self-assembly and pH-sensitivity of the prepared triblock copolymers when the length of PDEAEMA blocks is fixed. Meanwhile, the copolymer containing more hydrophilic PEG segments shows moderately lower drug loading capacity and smaller particle size. DOX release rates of the drug-loaded micelles can be controlled by the pH value and the PEG block length. Faster drug release rates are observed at lower pH values with shorter PEG block length. The MTT assay observation confirms the good biocompatibility of these pH-responsive copolymers. Therefore, the prepared pH-sensitive PDEAEMA65-PEGn-PDEAEMA65 triblock copolymers would be used as the promising candidate for anti-tumor drug carriers.
References
Börner HG (2009) Strategies exploiting functions and self-assembly properties of bioconjugates for polymer and materials sciences. Prog Polym Sci 34(9):811–851. https://doi.org/10.1016/j.progpolymsci.2009.05.001
Tyrrell ZL, Shen YQ, Radosz M (2010) Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers. Prog Polym Sci 35(9):1128–1143. https://doi.org/10.1016/j.progpolymsci.2010.06.003
Auriemma F, De Rosa C, Malafronte A, Di Girolamo R, Santillo C, Gerelli Y, Fragneto G, Barker R, Pavone V, Magilo O, Lombardi A (2017) Nano-in-Nano Approach for Enzyme Immobilization Based on Block Copolymers. ACS Appl Mater Interfaces 9(34):29318–29327. https://doi.org/10.1021/acsami.7b08959
Yang CX, Gao S, Dagnaes-Hansen F, Jakobsen M, Kjems J (2017) Impact of PEG Chain Length on the Physical Properties and Bioactivity of PEGylated Chitosan/siRNA Nanoparticles in Vitro and in Vivo. ACS Appl Mater Interfaces 9(14):12203–12216. https://doi.org/10.1021/acsami.6b16556
Tao YY, Zhao HY (2017) Synthesis and self-assembly of amphiphilic tadpole-shaped block copolymer with disulfides at the junction points between cyclic PEG and linear PS. Polymer 122:52–59. https://doi.org/10.1016/j.polymer.2017.06.046
Williford JM, Archang MM, Minn I, Ren Y, Wo M, Vandermark J, Fisher PB, Pomper MG, Mao HQ (2016) Critical Length of PEG Grafts on IPEI/DNA Nanoparticles for Efficient in Vivo Delivery. ACS Biomater Sci Eng 2(4):567–578. https://doi.org/10.1021/acsbiomaterials.5b00551
Cherng JY, Hou TY, Shih MF, Talsma H, Hennink WE (2013) Polyurethane-based drug delivery systems. Int J Pharm 450(1–2):145–162. https://doi.org/10.1016/j.ijpharm.2013.04.063
Ke F, Mo X, Yang R, Wang Y, Liang D (2009) Association of block copolymer in nonselective solvent. Macromolecules 42(14):5339–5344. https://doi.org/10.1021/ma900740b
Niu LY, Liu YY, Hou Y, Song WQ, Wang Y (2016) Self-assembly and micelle-to-vesicle transition from star triblock ABC copolymers based on a cyclodextrin core. Polym Chem 7(20):3406–3415. https://doi.org/10.1039/c6py00560h
Frisch H, Besenius P (2015) pH-switchable self-assembled materials. Macromol Rapid Commun 36(4):346–363. https://doi.org/10.1002/marc.201400623
Vuoriluoto M, Orelma H, Johansson LS, Zhu BL, Poutanen M, Walther A, Laine J, Rojas OJ (2015) Effect of Molecular Architecture of PDMAEMA-POEGMA Random and Block Copolymers on Their Adsorption on Regenerated and Anionic Nanocelluloses and Evidence of Interfacial Water Expulsion. J Phys Chem B 119(49):15275–15286. https://doi.org/10.1021/acs.jpcb.5b07628
Wang GY, Zhang LM (2016) Synthesis, self-assembly and pH sensitivity of PDEAEMA-PEG-PDEAEMA triblock copolymer micelles for drug delivery. React Funct Polym 107:1–10. https://doi.org/10.1016/j.reactfunctpolym.2016.08.001
Fabbri M, Soccio M, Costa M, Lotti N, Gazzano M, Siracusa V, Gamberini R, Rimini B, Munari A, Garcia-Fernandez L, Vazquez-Lasa B, San Roman J (2016) New fully bio-based PLLA triblock copoly(ester urethane)s as potential candidates for soft tissue engineering. Polym Degrad Stab 132:169–180. https://doi.org/10.1016/j.polymdegradstab.2016.02.024
Liu M, Leroux JC, Gauthier MA (2015) Conformation-function relationships for the comb-shaped polymer pOEGMA. Prog Polym Sci 48:111–121. https://doi.org/10.1016/j.progpolymsci.2015.03.001
Boudier A, Aubert-Pouessel A, Gerardin C, Devoisselle JM, Begu S (2009) pH-sensitive double-hydrophilic block copolymer micelles for biological applications. Int J Pharm 379(2):212–217. https://doi.org/10.1016/j.ijpharm.2009.05.032
Cai HH, Jiang GL, Shen ZH, Fan XH (2013) Solvent-induced hierarchical self-assembly of amphiphilic PEG(G(m))-b-PS dendritic-linear block copolymers. Soft Matter 9(m47):11398–11404. https://doi.org/10.1039/c3sm52439f
Phan H, Minut RI, McCrorie P, Vasey C, Larder RR, Krumins E, Marlow M, Rahman R, Alexander C, Taresco V, Pearce AK (2019) Role of self-assembly conditions and amphiphilic balance on nanoparticle formation of PEG-PDLLA copolymers in aqueous environments. J Polym Sci Pol Chem 57(17):1801–1810. https://doi.org/10.1002/pola.29451
Gil E, Hudson S (2004) Stimuli-reponsive polymers and their bioconjugates. Prog Polym Sci 29(12):1173–1222. https://doi.org/10.1016/j.progpolymsci.2004.08.003
Schmaljohann D (2006) Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev 58(15):1655–1670. https://doi.org/10.1016/j.addr.2006.09.020
Laskar P, Dey J, Ghosh S (2016) Evaluation of zwitterionic polymersomes spontaneously formed by pH-sensitive and biocompatible PEG based random copolymers as drug delivery systems. Colloids Surf B 139:107–116. https://doi.org/10.1016/j.colsurfb.2015.11.042
Oh KT, Kim D, You HH, Ahn YS, Lee ES (2009) pH-sensitive properties of surface charge-switched multifunctional polymeric micelle. Int J Pharm 376(1–2):134–140. https://doi.org/10.1016/j.ijpharm.2009.04.021
Manzanares-Guevara LA, Licea-Claverie A, Paraguay-Delgado F (2018) Preparation of stimuli-responsive nanogels based on poly(N, N-diethylaminoethyl methacrylate) by a simple “surfactant-free” methodology. Soft Mater 16(1):37–50. https://doi.org/10.1080/1539445x.2017.1391845
Farah S, Aviv O, Laout N, Ratner S, Beyth N, Domb AJ (2015) Quaternary ammonium poly(diethylaminoethyl methacrylate) possessing antimicrobial activity. Colloid Surface B 128:608–613. https://doi.org/10.1016/j.colsurfb.2015.01.051
Park JS, Lim YB, Kwon YM, Jeong B, Choi YH, Kim SW (1999) Liposome fusion induced by pH-sensitive copolymer: Poly(4-vinylpyridine-co-N, N '-diethylaminoethyl methacrylate). J Polym Sci Pol Chem 37(14):2305–2309. https://doi.org/10.1002/(Sici)1099-0518(19990715)37:14<2305::Aid-Pola3>3.3.Co;2-X
Tian W, Lv XY, Huang LB, Ali N, Kong J (2012) Multiresponsive Properties of Triple-Shell Architectures with Poly(N, N-diethylaminoethyl methacrylate), Poly(N-vinylcaprolactam), and Poly(N, N-dimethylaminoethyl methacrylate) as Building Blocks. Macromol Chem Phys 213(23):2450–2463. https://doi.org/10.1002/macp.201200283
Zhang Y, Ang CY, Li M, Tan SY, Qu Q, Luo Z, Zhao Y (2015) Polymer-Coated Hollow Mesoporous Silica Nanoparticles for Triple-Responsive Drug Delivery. ACS Appl Mater Interfaces 7(32):18179–18187. https://doi.org/10.1021/acsami.5b05893
Wu ZZ, Gan ZY, Chen B, Chen F, Cao J, Luo XL (2019) pH/redox dual-responsive amphiphilic zwitterionic polymers with a precisely controlled structure as anti-cancer drug carriers. Biomater Sci 7(8):3190–3203. https://doi.org/10.1039/c9bm00407f
Feng JJ, Wen WQ, Jia YG, Liu SL, Guo JW (2019) pH-Responsive Micelles Assembled by Three-Armed Degradable Block Copolymers with a Cholic Acid Core for Drug Controlled-Release. Polymers 11(3):511. https://doi.org/10.3390/polym11030511
Li S, Cai YY, Cao J, Cai MT, Chen YW, Luo XL (2017) Phosphorylcholine micelles decorated by hyaluronic acid for enhancing antitumor efficiency. Polym Chem 8(16):2472–2483. https://doi.org/10.1039/c6py02032a
Cortez-Lemus NA, Garcia-Soria SV, Paraguay-Delgado F, Licea-Claverie A (2017) Synthesis of gold nanoparticles using poly(ethyleneglycol)-b-poly(N, N-diethylaminoethylmethacrylate) as nanoreactors. Polym Bull 74(9):3527–3544. https://doi.org/10.1007/s00289-017-1906-5
Cohen N, Binyamin L, Levi-Kalisman Y, Berguig GY, Convertine A, Stayton P, Yerushalmi Rozen R (2016) pH and Salt Effects on Surface Activity and Self-Assembly of Copolymers Containing a Weak Polybase. Langmuir : the ACS journal of surfaces and colloids 32(36):9286–9292. https://doi.org/10.1021/acs.langmuir.6b02452
Adams DJ, Butler MF, Weaver AC (2006) Effect of block length, polydispersity, and salt concentration on PEO-PDEAMA block copolymer structures in dilute solution. Langmuir : the ACS journal of surfaces and colloids 22(10):4534–4540. https://doi.org/10.1021/la060192x
Li Y, Leng MT, Cai MT, Huang L, Chen YW, Luo XL (2017) pH responsive micelles based on copolymers mPEG-PCL-PDEA: The relationship between composition and properties. Colloid Surface B 154:397–407. https://doi.org/10.1016/j.colsurfb.2017.03.045
Tian M, Qin A, Ramireddy C, Webber SE, Munk P, Tuzar Z, Prochazka K (1993) Hybridization of block copolymer micelles. Langmuir : the ACS journal of surfaces and colloids 9(7):1741–1748. https://doi.org/10.1021/la00031a022
Maleki A, Zhu KZ, Pamies R, Schmidt RR, Kjoniksen AL, Karlsson G, Cifre JGH, de la Torre JG, Nystrom B (2011) Effect of polyethylene glycol (PEG) length on the association properties of temperature-sensitive amphiphilic triblock copolymers (PNIPAAM(m)-b-PEG(n)-b-PNIPAAM(m)) in aqueous solution. Soft Matter 7(18):8111–8119. https://doi.org/10.1039/c1sm05679d
Chen XR, Su Y, Shen F, Wan YH (2011) Antifouling ultrafiltration membranes made from PAN-b-PEG copolymers: Effect of copolymer composition and PEG chain length. J Membr Sci 384(1–2):44–51. https://doi.org/10.1016/j.memsci.2011.09.002
Ayen WY, Chintankumar B, Jain JP, Kumar N (2011) Effect of PEG chain length and hydrophilic weight fraction on polymersomes prepared from branched (PEG)(3)-PLA co-polymers. Polym Adv Technol 22(1):158–165. https://doi.org/10.1002/pat.1742
Wang JZ, del Rosario LS, Demirdirek B, Bae A, Uhrich KE (2009) Comparison of PEG chain length and density on amphiphilic macromolecular nanocarriers: Self-assembled and unimolecular micelles. Acta Biomater 5(3):883–892. https://doi.org/10.1016/j.actbio.2008.10.019
Matsumoto M, Takenaka M, Sawamoto M, Terashima T (2019) Self-assembly of amphiphilic block pendant polymers as microphase separation materials and folded flower micelles. Polym Chem 10(36):4954–4961. https://doi.org/10.1039/c9py01078e
Dong YM, Du PC, Pei ML, Liu P (2019) Design, postpolymerization conjugation and self-assembly of a di-block copolymer-based prodrug for tumor intracellular acid-triggered DOX release. J Mater Chem B 7(37):5640–5647. https://doi.org/10.1039/c9tb01511f
Verkoyen P, Johann T, Blankenburg J, Czysch C, Frey H (2018) Polymerization of long chain alkyl glycidyl ethers: a platform for micellar gels with tailor-made melting points. Polym Chem 9(44):5327–5338. https://doi.org/10.1039/c8py01312h
Zhang PY, Zhang ZH, Wang DL, Hao JC, Cui JW (2020) Monodispersity of Poly(ethylene glycol) Matters for Low-Fouling Coatings. ACS Macro Lett 9(10):1478–1482. https://doi.org/10.1021/acsmacrolett.0c00557
Wu B, Zhang L-J, Zhang C-J, Deng K, Ao Y-W, Mei H, Zhou W, Wang C-X, Yu H, Huang S-W (2020) Effect of Poly(ethylene glycol) (PEG) Surface Density on the Fate and Antitumor Efficacy of Redox-Sensitive Hybrid Nanoparticles. ACS Biomater Sci Eng 6(7):3975–3983. https://doi.org/10.1021/acsbiomaterials.0c00516
Miteva M, Kirkbride KC, Kilchrist KV, Werfel TA, Li H, Nelson CE, Gupta MK, Giorgio TD, Duvall CL (2015) Tuning PEGylation of mixed micelles to overcome intracellular and systemic siRNA delivery barriers. Biomaterials 38:97–107. https://doi.org/10.1016/j.biomaterials.2014.10.036
Jiang X, Li Y, Lu G, Huang X (2013) A novel poly(N-vinylcaprolactam)-based well-defined amphiphilic graft copolymer synthesized by successive RAFT and ATRP. Polym Chem-Uk 4(5):1402–1411. https://doi.org/10.1039/c2py20933k
Wu W, Zhang QJ, Wang JT, Chen M, Li S, Lin ZF, Li JS (2014) Tumor-targeted aggregation of pH-sensitive nanocarriers for enhanced retention and rapid intracellular drug release. Polym Chem-Uk 5:5668–5679. https://doi.org/10.1039/C4PY00575A
Wang K, Luo GF, Liu Y, Li C, Cheng SX, Zhuo RX, Zhang XZ (2012) Redox-sensitive shell cross-linked PEG–polypeptide hybrid micelles for controlled drug release. Polym Chem-Uk 3(4):1084–1090
Tian HY, Guo ZP, Lin L, Jiao ZX, Chen J, Gao SQ, Zhu XJ, Chen XS (2014) pH-responsive zwitterionic copolypeptides as charge conversional shielding system for gene carriers. J Control Release 174:117–125
Yang YQ, Zheng LS, Guo XD, Qian Y, Zhang LJ (2011) pH-sensitive micelles self-assembled from amphiphilic copolymer brush for delivery of poorly water-soluble drugs. Biomacromol 12(1):116–122
Acknowledgements
The authors are grateful for the support from Shanghai Branch, Du Pont China Co. Ltd. The authors wish to thank Prof. Dr. Hongli Zhao, Prof. Dr. Yun Ding and Prof. Dr. Aiguo Hu for their kindly assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, L., Zhang, C., Gu, X. et al. Self-assembly, pH-responsibility and controlled release of doxorubicin of PDEAEMA-PEG-PDEAEMA triblock copolymers: effects of PEG length. J Polym Res 28, 175 (2021). https://doi.org/10.1007/s10965-021-02532-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-021-02532-y