Abstract
A novel hyperbranched polyphosphate ester (HPE) was synthesized via the polycondensation of bisphenol-A and phosphoryl trichloride. The formed HPE was characterized by FTIR, 1H NMR and 31P NMR to confirm its structure. Then, a series flame retardant epoxy resins from bisphenol-A epoxy cured with HPE and bisphenol-A were prepared. The combustion behavior of the flame retardant epoxy resins was studied using limiting oxygen index (LOI) and cone calorimeter test. The LOI value increased from 23 to 32 when HPE, instead of bisphenol-A, was used as a curing agent. The cone calorimeter test data revealed that the cured bisphenol-A epoxy resin with HPE as a curing agent possessed improved flame retardancy. The photo graphs and scanning electron microscopy (SEM) of char residues confirmed the cone calorimeter results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epoxy resins are among the most important materials in modern polymer industries because of their balance of excellent heat, solvent, moisture, and chemical resistance, good mechanical and electrical properties, and satisfactory adherence to many substrates [1, 2]. On account of these characteristics, epoxy molding compounds are widely used for encapsulating semiconductor devices. The multifunctionalities of both epoxy and curing agent lead to highly crosslinking resins in microelectronic fields. However, as compensation for the flammability of the epoxy resins, brominated compounds and antimony oxide are imparted into the encapsulant composition as flame retardants. The utilization of these halogenated and antinomy compounds in polymeric materials is harmful to the environment and human health because toxic and corrosive gases are released as well as harmful endocrine-disrupting chemicals [3, 4]. Therefore, the trend is toward using halogen-free flame retardants in polymers [5–7]. Among the halogen-free flame retardants, phosphorus-containing retardants have demonstrated good flame retardancy for epoxy resins and also been found to generate less toxic gas and smoke than halogen-containing retardants [8].
Flame retardants are classified as additive-type and reactive-type. Additive-type flame retardants are present as fillers, while reactive-type flame retardants are introduced into resin systems through chemical reactions. It has been proved that reactive-type flame retardants as components of resins are more effective in fire retardant behaviour compared with those as additives incorporated into resins [9]. Moreover, the addition of large amounts of additive-type flame retardants decreases the mechanical properties, and results in some problems with processability.
Hyperbranched polymers have received considerable attention in recent years due to their unique architecture and properties [10–12]. Although hyperbranched polymers do not have perfectly defined structure, they retain many of the important features of dendritic polymers such as large number of end groups, offering the possibility for further modification, generally lower intrinsic viscosity in comparison with those of linear polymers of similar structures, and very good solubility characteristics, etc.; however, their preparation is more facile and easier to scale-up than dendrimers. Hyperbranched polymers containing phosphorus used as flame retardants have received a considerable interests [13, 14].
In this work, we report the synthesis of a hyperbranched polyphosphate ester (HPE) used as a reactive-type flame retardant form bisphenol-A and phosphoryl trichloride. The final product was characterized by FTIR, 1H NMR and 31P NMR. The flame retardancy of bisphenol-A epoxy resin cured with HPE were investigated by limiting oxygen index (LOI), cone calorimeter test, photo graphs, and scanning electron microscopy (SEM) measurements, respectively.
Experimental
Materials
Bisphenol-A (BA) was used as received. Phosphoryl trichloride (POCl3) was distilled prior to use. N-Methylpyrrolidinone (NMP) and pyridine were dried over CaH2 and distilled before use. Triphenyl phosphine (Ph3P) was used as a curing accelerator. All chemicals mentioned above were purchased from Shanghai First Reagent Co., China. Diglycidyl ether of bisphenol-A [SM618, epoxy equivalent weight (EEW) = 196 g/equiv] was obtained from Jiangsu Sanmu Group. (Yixing, China).
Synthesis of HPE [14]
POCl3 (6.43 g, 0.042 mol) was dissolved in 100 ml of freshly distilled NMP in a 500 ml, thoroughly dried, three-necked flask equipped with a reflux condenser, an over-head mechanical stirrer, and an additional funnel. BA (14.36 g, 0.063 mol) and pyridine (9.96 g, 0.126 mol) were completely dissolved in 200 ml of freshly distilled NMP with slight heating. After a homogeneous solution was obtained, the solution was slowly added to the reaction flask over the period of 1 h at ambient temperature. The reaction flask was then immersed in an oil bath preheated to 100°C, and the mixture was stirred vigorously for 10 h. The reaction solution was then cooled to room temperature, poured into methanol, filtered, re-dissolved in NMP, and washed repeatedly with water. The polymer was precipitated from methanol, filtered, and dried at 40°C in a vacuum oven for 24 h to give a white, powdery solid. The synthesis route of HPE is shown in Scheme 1.
IR (KBr): 1298 cm−1, stretching vibration of P = O; 1226, 1177 and 962 cm−1, stretching vibration of P-O-C (aromatic); 3200–3500 cm−1, stretching vibration of Ph-OH; 1613 and 1513 cm−1, vibration of benzene.
1H NMR (300 MHz, DMSO-d 6 , δ): 1.50 (-CH3), 4.07 (P-O-CH3), 7.0-7.3 (Ph-H), 9.05 (−OH).
Curing procedure for epoxy resins
HPE, BA, and their blends in the ratios of 33/67, 67/33 were used as curing agents. SM618 was mixed separately with above complexes at 100°C. Ph3P (total amount of HPE and BA) of 0.2 wt.% was added to each formulation as a curing accelerator. The mixtures were poured into hot aluminum moulds, and then thermally cured at 130°C for 1 h, followed by 170°C for 3 h and further postcured at 200°C for 3 h to obtain cured specimens. The cured specimens were denoted as HPE0/BA100, HPE33/BA67, HPE67/BA33, and HPE100/BA0.
Measurements
FTIR spectra
FTIR spectrum was recorded with a IR Prestige-21 spectrometer from Shimadzu Corporation, Japan.
1H and 31P NMR
1H and 31P NMR spectra were obtained with a Bruker Avance 300 spectrometer operating at 300 and 121.5 MHz, respectively, using dimethyl sulfoxide-d 6 (DMSO-d 6 ) as a solvent.
Limiting oxygen index (LOI)
Limiting oxygen index (LOI) was measured according to ASTM D2863. The apparatus used was an HC-2 oxygen index meter (Jiangning Analysis Instrument Company, China). The specimens used for the test were of dimensions 100 × 6.5 × 3 mm3.
Cone calorimeter test
The cone calorimeter (Stanton Redcroft, UK) tests were performed according to ISO 5660 standard procedures. Each specimen of dimensions 100 × 100 × 3 mm3 was wrapped in aluminium foil and exposed horizontally to an external heat flux of 35 kW/m2.
Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) studies were performed on the char residue using a Hitachi X650 scanning electron microscope.
Differential scanning calorimetry (DSC)
The differential scanning calorimetry (DSC) thermograms were recorded with a TA Shimadzu DSC-50 instrument at a heating rate of 10°C/min under nitrogen atmosphere.
Results and discussion
Characterization of HPE
In this study, the specific conditions for the polycondensation of BA and POCl3 monomers in the absence of gelation were determined according to the literature [14]. The initial molar ratio of BA to POCl3 was set to be 1.5:1. The final product bore both phosphoryl chloride groups and phenol groups at the terminals, and the latter are capable of curing epoxy resins. Methanol was used as a precipitator and to react with the remaining phosphoryl chlorides (Figs. 1 and 2).
The outcomes obtained from 31P NMR spectra demonstrated the reaction mechanism. Figure 3 displays the 31P NMR spectrum of a typical HPE prepared with a 1.5:1 molar ratio of BA to POCl3. Three signals at −5.8, −11.6 and −16.6 ppm are assigned to terminal, linear and dendritic units, respectively, on the basis of a comparison with previous work by Wang and co-workers [14]. Furthermore, 31P NMR spectra of the final products obtained at different reaction times are shown in Fig. 3. Comparing the peak area ratio of three characteristic signals, the polymers formed early in the reaction were essentially linear for 2 h. The signal corresponding to P in the dendritic units becomes more evident in the sample obtained from 6 h reaction. After 10 h of reaction, the ratio of dendritic units to linear units to terminal units is approximately 1:2:1. The content of dendritic units in the resulted hyperbranched polymer further increases at 14 h. These data indicate that the reaction of the third phosphoryl chloride group with phenol group is more difficult than the first two phosphoryl chloride groups in POCl3. Dendritic units only appear and gradually increase at a very high conversion.
Limiting oxygen index (LOI)
The LOI value can be used as an indicator to evaluate flame retardancy of a polymer. LOI is defined as the minimum fraction of oxygen in an oxygen-nitrogen mixture that is just sufficient to sustain combustion of the specimen after ignition. Thus, the flame retardant properties of these cured epoxy resins were further examined by measuring the LOI, and the results are also listed in Fig. 4. It is demonstrated that a higher LOI value is obtained with higher HPE content. It increases drastically from 23 to 27.5 by the incorporation of 33 wt.% HPE in the curing agent complex. The increase of LOI is linear with the content of HPE, which can be explained that HPE in the curing agent complex is able to form crosslinking char as a protective layer on the polymer surface during degradation.
Cone calorimeter test
Cone calorimeter test is an effective approach to compare the combustion behavior of flame retardant polymers. Heat release rate (HRR) results are shown in Fig. 5. The presence of HPE in cured epoxy sample decreases the HRR values significantly compared with cured epoxy sample by only BA (the HRR peak value of the latter is 1241.8 kW/m2). In the case of the cured epoxy sample with HPE, their peak HRR is behind that of the sample HPE0/BA100, and their peak values is much lower compared with HPE0/BA100. The peak values of the samples with HPE are 491.8, 388.1, and 306.8 kW/m2, respectively. Furthermore, the HRR curves of the samples with HPE are very smooth. The above phenomenon can be explained by the compact char residue formed on the surface of the sample in the cone calorimeter test (Fig. 13). However, it is noted that the ignition time of the cured epoxy samples with HPE is less than that of HPE0/BA100. The reason is due to the fact that phosphate ester decomposes at low temperature after the cone heater irradiated the surface of the composite, and some small volatile molecules are produced from the decomposition of HPE [15].
It can be seen in Fig. 5 that the HRR curve of the cured epoxy samples with HPE is very flat and the values of HRR decrease further with the addition of HPE Moreover, addition of HPE strongly prolongs the process of combustion compared with HPE0/BA100. From the above results, it can be concluded that the addition of HPE can remarkably enhance the flame retardant properties of cured epoxy samples.
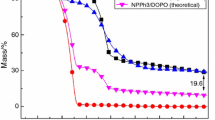
The primary parameter which was responsible for HRR of the samples with HPE is the mass loss rate (MLR) during combustion, which was significantly reduced compared with those values observed for the sample without HPE. Figure 6 shows that the MLR decreased in the order of HPE100/BA0>HPE67/BA33>HPE33/BA67>HPE0/BA100, this trend is the same as those of the HRR in the cone calorimeter (Fig. 5). This phenomenon also can be illustrated using the photo graphs after cone calorimeter test (Fig. 13).
Figure 7 shows the weight of the char residues. It can be seen from Fig. 7, the char residue weight of samples containing HPE is higher than that of HPE0/BA100 without HPE. In case of the samples with HPE, a compact char residue will be formed during combustion. It has been reported that phosphate ester can decompose at low temperature to form char residue[15]. There is compact char residue formed on the surface of the samples with HPE between 50s and 150 s in the cone calorimeter test. The physical process of the char residue would act as a protective barrier and can thus limit the oxygen diffusion to the substrate or give a less disturbing low volatilization rate.
Figure 8 presents the total heat release (THR) for all samples. The slope of THR curve can be assumed as representative of fire spread [16]. From Fig. 8, the THR of the sample containing HPE is lower than the sample without HPE, which may be the fact that there is much char residue formed in the case of the sample with HPE (Fig. 7). With the addition of HPE, the THR decreases. It also can be seen that the THR of HPE100/BA0 is the lowest one among all the samples in the time between 0 to 575 s. That is, the flame spread of HPE100/BA0 is comparatively the lowest among all the samples. In the combustion process, there is condense and compact char residue formed, which can isolate heat from the outside and combustible gases from the inside. For the HPE100/BA0, the structure of char is good enough to stop flame spread. It is also suggested HPE shows good flame retardance for bisphenol-A epoxy.
Figure 9 shows the Effective heat of combustion (EHC) values of the tested samples. The maximum EHC value decreases with the addition of HPE, but the time to reach the maximum EHC of cured epoxy with HPE is longer than that of cured epoxy without HPE. After adding HPE to the system, the maximum EHC becomes smaller. The decrease in the EHC value of the cured epoxy samples with HPE could be ascribed to the following factors. The samples, HPE existing in the systems, release less heat when they decompose or burn on heating than the sample without HPE. It should be figured out that the curves of all the samples are not smooth, the reason may be that there is some holes or gaps on the surface of char residue (Fig. 13). The uniform release of combustible gas from the underlying materials leads the flutter of the curves.
Smoke performance of flame retardant material is a very important parameter in fire safety fields. Smoke extinction area (SEA), which represents the relationship between the volatile property and smoke emission, is another very valuable parameter [17]. It can be observed from Fig. 10 that the samples with HPE released higher quantities of smoke from volatiles than the sample without HPE during the time between 0 s and 100 s. Furthermore, the quantities of smoke released from the samples with HPE increases with the addition of HPE. This may be attributed to the decomposition of phosphate ester at low temperature to form some aromatic structure from HPE; aromatic compounds produce more smoke in a fire. It should be figured out the time of releasing smoke extends with the addition of HPE. This can be illustrated by the fact that there are some compact char residue formed on the surface of the samples with HPE. The increase of released smoke together with char residues during burning results is not sufficient flaming of the samples with HPE. This is the main reason why THR, HRR, and EHC are reduced greatly. However, the increase of SEA can be avoided by introducing smoke suppressant into flame retardant epoxy systems to improve the strength of the char residue layer formed during the burning process [18].
The smoke produce rate (SPR) values of bisphenol-A epoxy cured with BA and HPE are illustrated in Fig. 11. The peak SPR value of HPE0/BA100 was the highest one (0.466 m2/s) among all the samples. The peak SPR value decreased greatly with the addition of HPE; and, when the loading of HPE is 33 wt% of all the curing agent, the peak SPR value of HPE33/BA67 is 0.201 m2/s. Furthermore, the SPR value of the samples with HPE decreases with the addition of HPE. The time to the peak SPR value of HPE33/BA67 (350 s) was longer than that for HPE0/BA00 (220 s). However, when the loading of HPE is raised to 67 wt% and 100 wt% of all the curing agent, the times to the peak SPR value of HPE67/BA33 and HPE100/BA0 are deceased to 55 s and 45 s, respectively. The results imply that moderate content of HPE produces obvious smoke suppression in the flame retardant epoxy system by decreasing the peak SPR value, and delay time to the peak SPR value. Smoke suppression by HPE can be explained as follows: HPE can promote charring and enhance the quality of char, which can protect the inner matrix and reduce the amount of combustible gas and smoke-forming materials in the gas phase during combustion. In the case of HPE67/BA33 and HPE100/BA0, phosphate ester at high loading decompose at low temperature produce much smoke at the beginning of the cone calorimeter test.
The total smoke release (TSR) values of bisphenol-A epoxy cured with BA and HPE are illustrated in Fig. 12. The TSR value of HPE0/BA100 at combustion termination was greatest among all the samples. The TSR values of the samples with HPE decrease with the addition of HPE at combustion termination. This indicates that the total amount of smoke decreased when the HPE was introduced into flame retardant epoxy system. However, in the time between 70 s and 130 s, The TSR values of the samples with HPE are high than that of the sample without HPE. This phenomenon is due to the fact that phosphate ester group in HPE decomposes at low temperature.
Photo graphs of char residues
Figure 13 are digital photos of residues of the series of samples. It can be seen that a more coherent and dense char can be formed with the addition of HPE. From the char structure, we can explain the combustion phenomenon of the flame retardant epoxy samples. The formation of the efficient char can prevent the heat transfer between the flame zone and the burning substrate, and thus protect the underlying materials from further burning and retard the pyrolysis of polymers. As a result, HRR values are strongly reduced, as shown in Fig. 5. The residue of HPE100/BA0 was tighter, denser, and higher than any other residue, at the same time the residue of the samples with HPE were more dense and higher than HPE0/BA100. The results are in accordance with the order in Fig. 5. This can be explained that the HPE can lead to the formation of ceramic-like material with a homogeneous surface which will protect the material throughout combustion and also to a mechanical reinforcement of the charred layer which would lead to a better accommodation of strain. However, the microcomposite may lead to the formation of an inhomogeneous and thus brittle surface material. The different char residues also contribute to the flame retardancy performance. For the above reasons the HRR of the samples with HPE is lower than that of HPE0/BA100.
Scanning electron microscopy (SEM)
The expanding crust is usually formed in an intumescent system during combustion. To separate oxygen from the degraded volatiles more efficiently, the surface of the crust should be compact enough to prevent the penetration of gases.
The rapid formation of the protective char layer is highly dependent upon factors such as the fire temperature and the viscosity of the melted coating. An intumescent coherent char structure is observed in Fig. 14 (B’, C’, D’). The intumescent char structure can prevent the heat transfer between the flame zone and the underlying substrate, and thus protects the substrate from heat and fire. The transfer speed of heat through the char layer depends on the resistance of the substrate to fire, so the expanding effect and the formation of the intumescent char structure are very important to general fire resistant properties of the cured resins. Some cracks and large open holes in the char structure are presented in Fig. 14 (B’). This phenomenon can be attributed to low viscosity of the melted coating and low strength of the char structure. Although air in these large holes can lower heat transference, air convection increases the speed of heat transference. The intumescent structure is also observed in Fig. 14 (C’, D’). However, the char residues are more compact than that of HPE33/BA67. This phenomenon can be illustrated that the char residue increases with the addition of HPE.
Differential scanning calorimetry (DSC)
Glass transition temperature (T g ) is a very important parameter for epoxy resins and epoxy matrix composites because it establishes the service environment for the materials’ usage. It varies widely with the structures, molecular weights and other thermodynamic parameters of the polymers, such as intermolecular force and chain flexibility [19]. It can be found that the T g of the cured film of epoxy resin with HPE (135.3°C) is higher than that cured with BA (128.7°C) in the present study (Fig. 15). This can be illustrated by the effect of crosslinking density, which can increase T g of the cured film [20]. The equivalent ratio between phenol and epoxy groups is 1:1 when preparing the epoxy resin systems using HPE and BA, separately. However, HPE is a multifunctional compound, which acts as a crosslinker, resulting in a much higher crosslinking density. The effect of crosslinking density to T g is greater than that of chain flexibility, leading to a higher T g of the epoxy resin cured with HPE.
Conclusions
A novel hyperbranched phosphate ester (HPE) with high functionality has been synthesized, which act as a curing agent of epoxy resin and can improve the T g of the final product.
The flame retardance of cured bisphenol-A materials increased with increasing HPE content. Incorporating 33 wt.% HPE in the curing agent complex into flame retardant epoxy material achieved an LOI value of 27.5. The results of the cone calorimeter tests indicated that with the increasing HPE, the HRR, THR, EHC decreased greatly, whereas the char residue amount and SEA values increased with increasing HPE content. The photographs and SEM results after cone calorimeter test indicates that, for char residues, the carbon layer became more compact and whole with increasing HPE content.
References
Hodgkin JH, Simon GP, Varley RJ (1998) Thermoplastic toughening of epoxy resins: a critical review. Polym Advan Technol 9:3–10
Chen WY, Wang YZ, Chang FC (2004) Thermal and flame retardation properties of melamine phosphate-modified epoxy resins. J Polym Res 11:109–117
Levchik SV, Weil ED (2004) Thermal decomposition, combustion and flame-retardancy of epoxy resins-a review of the recent literature. Polym Int 53:1901–1929
Dumler R, Thoma H, Lenoir D et al (1989) Thermal formation of polybrominated dibenzodioxins (PBDD) and dibenzofurans (PBDF) from bromine containing flame retardants. Chemosphere 19:305–308
Green J (1992) A review of phosphorus-containing flame retardants. J Fire Sci 10:470–487
Chen L, Wang YZ (2010) A review on flame retardant technology in China. Part I: development of flame retardants. Polym Advan Technol 21:1–26
Weil ED, Levchik S (2004) A review of current flame retardant systems for epoxy resins. J Fire Sci 22:25–40
Wang CS, Shieh JY (1998) Synthesis and properties of epoxy resins containing 2-(6-oxid-6H-dibenzc, e 1,2oxaphosphorin-6-yl)1,4-benzenediol. Polymer 39:5819–5826
Price D, Pyrah K, Richard Hull T et al (2002) Flame retardance of poly(methyl methacrylate) modified with phosphorus-containing compounds. Polym Degrad Stabil 77:227–233
Inoue K (2000) Functional dendrimers, hyperbranched and star polymers. Prog Polym Sci 25:453–571
Jikei M, Kakimoto M (2001) Hyperbranched polymers: a promising new class of materials. Prog Polym Sci 26:1233–1285
Gao C, Yan D (2004) Hyperbranched polymers: from synthesis to applications. Prog Polym Sci 29:183–275
Deng J, Zhu SW, Shi WF (2004) Effect of molecular chain structure of the cured epoxy resin containing hyperbranched (3-hydroxyphenyl) phosphate on expansion and flame retardance. J Appl Polym Sci 94:2065–2070
Wang QF, Shi WF (2006) Synthesis and thermal decomposition of a novel hyperbranched polyphosphate ester used for flame retardant systems. Polym Degrad Stabil 91:1289–1294
Wang X, Hu Y, Song L et al (2010) Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer 51:2435–2445
Almeras X, Le Bras M, Hornsby P et al (2003) Effect of fillers on the fire retardancy of intumescent polypropylene compounds. Polym Degrad Stabil 82:325–331
Manfredi LB, Rodríguez ES, Wladyka-Przybylak M et al (2006) Thermal degradation and fire resistance of unsaturated polyester, modified acrylic resins and their composites with natural fibres. Polym Degrad Stabil 91:255–261
Pereira CMC, Herrero M, Labajos FM et al (2009) Preparation and properties of new flame retardant unsaturated polyester nanocomposites based on layered double hydroxides. Polym Degrad Stabil 94:939–946
Lee SC, Min BG (1999) Depression of glass transition temperature due to the chain extension in glassy state. Polymer 40:5445–5448
Liu YL (2002) Epoxy resins from novel monomers with a bis-(9,10-dihydro-9-oxa-10-oxide-10-phosphaphenanthrene-10-yl-) substituent. J Polym Sci Part A-Polym Chem 40:359–368
Acknowledgments
The authors gratefully acknowledge the Outstanding Young Scientist Research Award Fund from Shandong Province (BS2009CL015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, X., Jiao, C., Li, S. et al. Flame retardant epoxy resins from bisphenol-A epoxy cured with hyperbranched polyphosphate ester. J Polym Res 18, 2229–2237 (2011). https://doi.org/10.1007/s10965-011-9636-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-011-9636-0