Abstract
Melamine cyanurate (MCA) flame retardant polyamide 6 (PA6) shows good flame retardancy, but the corresponding mechanisms have not been completely understood. In this paper, Fourier transform infrared spectra (FTIR), elemental analysis (EA), scanning electronmicroscope (SEM), energy dispersive scanning (EDS), thermogravimeric analysis (TGA) and pyrolysis-gas chromatogram-mass spectrometer (Py-GC-MS) were conducted to investigate the processes including melt-drip phase, gaseous phase and condensed phase of MCA/PA6 system. Compared with original PA6, it is found that MCA flame retardant PA6 mainly undergoes predominant weak bond-breakage degradation forming oligomers rather than oxidative degradation producing low-boiling point fuel as original PA6 does. The produced oligomers can accelerate the formation of the melt drips which effectively removes the combustion heat and latent fuel, also the self-condensation of these oligomers is advantageous to form stable cross-linking structure, thus greatly consolidating the char layer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
PA6 is an important engineering resin manufactured in large quantities worldwide due to its excellent mechanical properties, good wear resistance, electricity and oil proof performance. But like most polymers, original PA6 is flammable with LOI of only 23, thus restricting its application in some important fields, particularly in electrical industries for various parts such as clip fasteners, switch components, wire ties, electrical connectors, terminal blocks, etc., therefore, flame retardant PA6 is badly in need.
In recent years, traditional halogen flame retardants have been challenged as the results of the ecological problems, and halogen-free products are playing more and more important roles [1–6]. Among the halogen-free flame retardants applied in PA6, MCA shows some remarkable advantages including high nitrogen content, satisfactory flame retardancy, non-corrosion and innocuity. Although the applications of MCA in flame retardant PA6 were reported early in 1970’s [7–10], the detailed investigations on its mechanism have been published very little. The present accepted viewpoints emphasized that the MCA experiences a endothermic decomposition into melamine and cyanurate above 350°C, and this process is advantageous to decrease the temperature of the burning material. Meanwhile, MCA can accelerate the pyrolysis of PA6 to form nonflammable drips, thus leading to quick self-extinguishment of the burning material. However, just as Casu A. recognized, the action of melamine cyanurate in polyamide was not completely understood [11], and he concluded that the MCA involved a strong condensed-phase mechanism which was not limited to previous accepted “drip promoter” process. Levchik S.V. calculated [12] the decomposition activation energy of original PA6 and MCA/PA6 systems from the TG data obtained at different heating rates. It was found the activation energy was higher and progressively increased with the advancement of the decomposition reaction for original PA6, indicating the process was complex, however, MCA flame retardant PA6 showed two separated steps with lower activation energy, which showed that the interaction between PA6 and MCA resulted in an accelerated thermal decomposition due to the formation of more stable intermediate compound. These investigations were significant to reveal the fire-resistance mechanism of PA6/MCA system, however, seemed still not enough. Because the thermal-oxidative degradation of flame retardant PA6 has a complicated process, with the condensed phase, gaseous phase and melt-drip phase involved, therefore only systemically investigating above three processes rather than single one, the more exact mechanisms can be further revealed.
In this investigation, FTIR, EA, SEM, EDS and GC-MS analysis were done to study the three processes, thus comprehensively revealing the flame retardancy mechanisms of MCA flame retardant PA6.
Experimental
Materials
Melamine cyanurate: powder product, Chengdu Kelong Chemical Reagent Company, China.
PA6: granulated product (MFI: 10.1 g/10 min) of Yueyang Petro-Chemical Cooperation, China.
Preparation of flame retardant PA6
Pre-mix 7 Wt % MCA and 93 Wt % original PA6 granulate in high-speed mixer, then extruded the mixture to granulates by twin-screw extruder (ϕ: 30 mm, L/D: 32, model: SLJ-30, Longchang Chemical Engineering Equipment Company , China), finally molded into flame retardant PA6 samples by injector (K-TEC 40, Terromatik Milacron Corporation, Germany).
Characterization
The LOI was measured by an ATLAS limiting oxygen index instrument with 120 × 6.5 × 3 mm bars according to ASTM D2863-70. The vertical burning experiment was conducted by a CZF-3 horizontal and vertical burning tester with 127 × 12.7 × 1.6 mm bars according to UL-94 test ASTM D3801. FTIR spectra of the components in the melt-drip phase of MCA flame retardant PA6 and original PA6 were recorded using a Nicolet 20SXB FTIR spectrometer, and their EA were conducted by CARVO ERBA 1106 elemental analyzer. Py-GC-MS analysis of the decomposition products of the gaseous phase of MCA flame retardant PA6 and original PA6 were measured by HP5890SA Py-GC-MS spectrometer (pyrolysis temperature: 550°C). The residual char layer of MCA/PA6 and original PA6 materials through thermal-oxidation degradation in oven (1 h at 400°C) were observed by a HITACHI X-650 SEM instrument. EDS analysis of the residual char layer of the burned MCA flame retardant PA6 and original PA6 were done by JEOL JSM-5900LV EDS analyzer. TGA of the above materials were conducted by General V 4.1C DuPont 2100 DTA thermal analyzer at a constant heating rate (10°C/min) in the temperature range of 25~580°C and in atmosphere flow.
Results and discussion
The incorporation of MCA (7%) can greatly improve the flame retardancy of PA6. Our test shows that the LOI and UL94 of MCA flame retardant PA6 can be enhanced to 31 and V0 rating respectively (the LOI and UL94 rating of original PA6 are 23 and V2 rating). The mechanism of the remarkable enhancement of the flame retardancy by introducing MCA were investigated through analyzing melt-drip phase, gaseous phase and condensed phase as follows.
Melt-drip phase analysis
The generation of melt-drip is typical phenomena for the combustion process of PA6. The main components in the melt-drip phase mainly involve the involatile decomposition products of polyamide materials, which can provide useful information concerning the pyrolysis process, however, ignored by previous investigations. In our studies, FTIR and EA were employed to study the difference of the components in the melt-drip phase of original PA6 and MCA /PA6.
Figure 1 provides the FTIR spectra of the components in the melt-drip phase of the above systems. It was observed that the main absorption for both spectra were in agreement. However, their relative strength showed obviously different. Particularly for 2925 and 2856 cm−1 attributed to the C-H stretching vibration (VC-H), their relative strength of original PA6 was far weaker than those of MCA flame retardant PA6. As the main characteristic absorption of hydrocarbon, VC-H strength can reveal the content of hydrocarbon-containing compounds in a system. Accordingly, it was concluded that the components in the melt-drip phase of the flame retardant PA6 had much higher hydrocarbon-containing compounds than original PA6. In addition, it was found that there is obvious absorption at 3314 cm-1 for MCA flame retardant PA6 system, but much weaker absorption at this wavenumber for original PA6 system. This absorption is attributed to N-H stretching vibration (VN-H) from cyanurate and caprolactam, the decomposed product of MCA and PA6 respectively. Obviously, the melt drip phase of MCA flame retardant PA6 contains higher N-H-containing components compared with ordinal PA6 system.
In order to confirm the above conclusion, EA of the melt-drip phase of both systems were conducted and it showed that the C and H percentage of MCA flame retardant PA6 in the melt-drip phase were 50.1% and 7.5%, obviously higher than those of original PA6, 40.5% and 6.2% respectively. The data further confirmed the FTIR analysis.
For a polymer like polyamide, its main decomposition products are various hydrocarbon-containing compounds, usually including the products with low-boiling point such as lower hydrocarbon and the corresponding monomers (such as caprolactam) produced by complete depolymerization, and the ones with high-boiling point such as oligomers by incomplete depolymerization. The decomposition reactions producing above two kinds of products simultaneously occur and compete with each other. During the burning process of PA6, the produced volatile hydrocarbon-containing compounds with low-boiling point rapidly vaporize into the gaseous phase as the fuel supporting combustion, but those involatile oligomers easily turn into the melt drip phase and quickly remove from the burning materials. According to higher content of hydrocarbon-containing compounds in melt-drip phase from FTIR and EDS analysis, it shows that hydrocarbon-containing components of MCA/PA6 system has less consumption compared with original PA6, illuminating the former experienced more oligomers producing process, which further accelerates the melt-drip process, thus not only taking away lot of latent fuel, but also a great deal of combustion heat from the bulk materials. Obviously, this process for MCA/PA6 is more advantageous to flame retardancy.
Gaseous phase analysis
As the results of numerous volatile degradation components of PA6 as well as their complicated chemical interaction, the gaseous phase analysis is complex. Py-GC-MS was adopted to investigate the process. The advantages of this method lie in that the relative content and molecular structure of the degradation products are available at one time.
From the decomposition products of original PA6 and MCA flame retardant PA6 in gas chromatography as shown in Fig. 2, it can be seen that a lot of peaks appeared, indicating rather complicated components involved. The following MS analysis ascertained that the main degradation components were caprolactam (A), cyclopentanone (B), butyronitrile (C) and valeronitrile (D) respectively, showing the same for both systems (Table 1).
However, the relative strength of their corresponding peaks (singed as A-D) in GC were obviously different. For original PA6 system, the caprolactam (A) and cyclopentanone (B) showed much stronger peaks than those of nitrile compounds. However, the relative strength of (A) and (B) sharply decreased and the peaks of nitrile compounds correspondingly enhanced for MCA/PA6 system. The obvious change of the degradation products in gaseous phase showed that MCA had great impacts on the PA6’s degradation process.
The decomposition of PA6 in atmosphere is a very complicated process including the oxidative degradation initiated by active free radicals and the degradation caused by weak bond-breakage on molecular chains, also including the self-isomerization of some instable intermediate products as well as their interaction to produce more complicated compounds. Therefore, it is hard to exactly describe the decomposition process of PA6. According to the main products identified by Py-GC-MS analysis and general degradation rules of polyamide, PA6 is likely to mainly undergo the two decomposition course as Scheme 1 and 2.
According to the above mechanisms, it is known that the typical products of the oxidative degradation of PA6 were caprolactam and cyclopentanone. However, for the degradation by weak bond-breakage, the degradation products not only involved caprolactam, but also more chain fragments with relatively long molecular chains such as nitrile compound. The fact that MCA/PA6 system has higher nitrile content, and lower caprolactam and cyclopentanone content in the gaseous phase, confirms that the incorporation of MCA in PA6 actually facilitates weak bond-breakage degradation. The mechanism was related to the interaction between PA6 chains and the decomposition products of MCA. As melamine molecules possessing amine groups can interfere with the hydrogen bonding network of PA6, it can decrease electron density at >C = O bond of PA6 chains and facilitate α-scission (CH2-CO) of PA6 chain. Otherwise, another decomposition product cynaurate has great impacts on the weak bond-breakage degradation of PA6. As is well known, cynaurate can form two kinds of isomer as the result of proton transfer, i.e., ketone form and enol form. It has proved that the content of ketone form is over 95%, much higher than that of enol form. Accordingly, the effect of the ketone form on the degradation of PA6 is dominating. This cyanurate of ketone form can accelerate weak bond-breakage through the interaction of its -C = O groups with the -NH- of PA6 leading to β-scission (NH-CH2) of PA6 chain.
Condensed phase analysis
The condensed phase process is an important aspect for the flame retardancy of polymers. However, for MCA/PA6 system, a gaseous phase mechanism rather than condensed one is more emphasized in the past investigations.
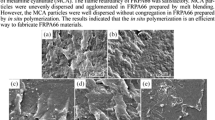
From the SEM photograph of residual char of original PA6 and MCA /PA6 (Fig. 3), it can be seen that there were many holes dispersed on the char surface for original PA6 due to insufficient char-formation on the burning materials surface. For MCA/PA6 system, almost no holes were observed, which illuminated that MCA can effectively consolidate the condensed phase process to a certain degree. Obviously, the closed char layer is advantageous to hold back the transfer of the heat, fuel and oxygen, thus favors the improvement of flame retardancy of the material. The TG analysis in Fig. 4 also proved this conclusion. The residual char weight ratio for PA6 is only 0.3%, however, 2.4% for MCA/PA6 system, much higher than that of the former. Further proof came from the EDS analysis with C, H and O percentage of the residual char. It was found that MCA flame retardant PA6 had higher C (44.9%) but lower O content (34.5%) respectively compared with the original PA6 (38.2% C and 45.5% O). These data offered valuable information because oxidation is an important process for the combustion of materials. Therefore, higher chemical combination degree with oxygen generally means a more intensive combustion process. From the O percentage of residual char, it can evaluate the fire-resistance of corresponding materials. The lower O percentage of the residual char of PA6/MCA system revealed the oxidation reaction greatly depressed with an imperfect combustion, which is likely related to the isolating effects of the closed char layer of MCA/PA6 system.
The reason of the condensed phase consolidated for MCA/PA6 system mainly results from the self-condensation of the produced oligomers, and this process can be described in Scheme 3. As the addition of MCA is more advantageous to weak bond-breakage degradation, there are more oligomers generated, thus more cross-linking structure as shown in Scheme 3 can be formed to effectively consolidate the char layer as compared to original PA6.In order to confirm the above mechanism, FTIR spectra of the condensed phase from MCA/PA6 and original PA6 as shown in Fig. 5. Both of the systems have absorption at 1656, 1553, 1441 and 1359 cm−1, which belong to the characteristic absorption of the remained PA6 chains. It can be seen that for MCA/PA6, there are absorption peak at 2249, 2159 and 818 cm−1 which are agreement with the characteristic absorption of nitrile, carbodiimide and isocyanurate [12], however, no obvious absorption was observed at this wavenumber for original PA6. The result of FTIR is a good support to the mechanism as shown in Scheme 3.
Conclusions
The studies investigated the mechanisms of MCA flame retardant PA6, and revealed that the incorporation of MCA had important influence on PA6’s degradation through the analysis with melt-drip phase, condensed phase and gaseous phase involved. It is concluded that the introduction of MCA more easily causes weak bond-breakage degradation through the interaction between amide groups of PA6 and melamine or cyanurate. Compared with original PA6 dominated by oxidation degradation to generate small molecular products, MCA/PA6 can produce more oligomers during the decomposition. These oligomers can accelerate the formation of melt drips which take away a great of combustion heat and latent fuel, and decrease the amount of volatile flammable components. Moreover, the self-condensation of these produced oligomers can from some stable cross-linking structure, thus consolidating the condensed phase and promoting the formation of the closed char layer, which can effectively isolate the heat, volatile decomposition products and oxygen. Therefore, condensed phase mechanism has great contribution to the improvement of the flame retardancy for MCA/PA6 system.
References
Sorathia U, Lynon R, Gann RG (1997) Fire Technol 33:260. doi:10.1023/A:1015371806854
Christy RM (1993) Plastics Compounding 16:56
Clarke FB (1999) Fire Mater 23:109. doi:10.1002/(SICI)1099-1018(199905/06)23:3<109::AID-FAM674>3.0.CO;2-W
Zhu XS, Xu HS, Lu JM, Wang JY, Zhou SH (2008) J Polym Res 15:195. doi:10.1007/s10965-007-9170-2
Felipe S, Félix G, José L, Peña L, Verónica C, José M (2007) J Polym Res 14:341. doi:10.1007/s10965-007-9112-z
Chen WY, Wang YZ, Chang FC (2004) J Polym Res 11:109. doi:10.1023/B:JPOL.0000031069.23622.bc
Casu A, Camino G (1998) Polym J 30:1. doi:10.1295/polymj.30.1
United States Patent 8639740
United States Patent 43149274
United States Patent 4317766
Casu A, Caimno G (1997) Polym Degrad Stabil 58:297. doi:10.1016/S0141-3910(97)00061-X
Levchik SV, Balabanovich AI, Levchik GF, Costa L (1997) Fire Mater 21:75. doi:10.1002/(SICI)1099-1018(199703)21:2<75::AID-FAM597>3.0.CO;2-P
Acknowledgements
The financial support from the National Natural Science Foundation of China (No. 50803039) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Wang, Q. The investigation on the flame retardancy mechanism of nitrogen flame retardant melamine cyanurate in polyamide 6. J Polym Res 16, 583–589 (2009). https://doi.org/10.1007/s10965-008-9263-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-008-9263-6