Abstract
This paper describes certain wholly aromatic polyamides based on 1,3-diamino-4-halobenzenes and 1,3-diamino-4,6-dihalobenzenes, and on isophthaloyl and terephthaloyl chloride by means of low temperature solution polymerization. We set out to study the influence of the kind of halogen (F, Cl or Br) and the type of substitution (mono or di) in the diamine moiety with regard to solubility, water uptake and thermal and mechanical properties. The materials are characterized with respect to chemical structure and purity by elemental analysis, infrared (FT-IR) and nuclear magnetic resonance (NMR) spectroscopic techniques, and special attention is paid to the sequence distribution (constitutional order) of polymers derived from non-symmetric monohalogen-substituted diamines, and their influence on the above-mentioned properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wholly aromatic polyamides are characterized by their excellent balance of thermal and mechanical properties, which makes them useful as high performance materials for advanced technologies in automotive, electrical, electronic, and other industrial markets. Thus, poly(p-phenylene terephthalamide) and poly(m-phenylene isophthalamide) have found application in high temperature-high strength fibres, coatings and fillers [1–3]. However, they encounter processing difficulties due to their extremely high transition temperatures, which lie over the decomposition temperatures, and due to their poor solubility in common organic solvents. However, in spite of their excellent balance of thermal and mechanical properties, their low chemical resistance to certain common chemicals such as chlorine, and their limited flame resistance have restricted their applications in some high technology fields, whence many efforts have been made to enhance their processability, solubility, thermal and chemical resistance by chemically modifying their structure through a variety of methods: for instance, by introducing bulky side groups [4–10], by incorporating flexible chains into the polyamide backbone [11–15], or by introducing groups that enhance mechanical and thermal resistance, i.e. fire resistance.
In this work we explore the property modifications of wholly aromatic polyamides by using meta-oriented or asymmetrically substituted monomers [16–22]. The introduction of these kinds of monomers, so that monomeric units become symmetrically non-equivalent, leads to constitutional isomerism due to the unequal reactivities of monomer unit functional groups, and the sequence distribution influences the final polyamide properties.
Theoretical constitution isomerism has been widely discussed in several papers by Odian [23], Suter et al. [24–27] and Ravindranath [28], and practical and theoretical aspects of sequence control in one-step condensation polymerism have also been described by different authors [18], and concisely summarized by Ueda [29]. The authors [30] of this paper have previously described the practical and theoretical aspects of the polymerization of 1,3-phenylenediamines asymmetrically substituted by a chlorine atom in ring position 4.
Structure-property relationships arising from constitutional isomerism are as yet not well known for polyamides, nevertheless their sequence distribution may lead to a partially disordered polymer structure that can have a strong effect on cohesive energy and crystallinity.
It has been found that aromatic polyamides are very sensitive to oxidation by chlorine, and that the ortho positions of the aromatic diamine compounds m-phenylenediamine were easily substituted by chlorine in the manner of an Orton rearrangement through N-chlorination [31], which decreased mechanical and thermal properties and also affected the specific properties of material applications, such as performance in reverse osmosis membranes in terms of flux rate and salt rejection. Konogaya et al. [32] have shown that the introduction of substituents into the ortho positions of p-phenylenediamine has a widespread effect on decreasing the chlorine uptake, which represents the first step of oxidation by chlorine. In particular, the largest effect, by far, on polymer protection against chlorine is due to the introduction of a mono chlorine substituent.
Wholly aromatic polyamides have been widely used in the fabrication of wide-bodied aircraft interiors and many of these applications have to be flame retardant, which means that any improvement in the flame-resistant behaviour of these materials is obviously very important. Their flame resistance is somewhat related to the amount of char formed on the thermal degradation of a polymer, and there is a significant correlation between char yield and the limiting oxygen index [33, 34]. Hence, the well-established effectiveness of halogen in polymer flame retardancy [35] as studied by Kakimoto et al. [36], whose work showed a great increase in the limiting oxygen index in halogen-substituted aromatic polyamides in comparison to poly(m-phenyleneisophthalamide). It is well known that wholly aromatic polyamides burn with difficulty forming black insulating char. Thus, the inclusion of halogens in the polymer structure increases flame resistance. This positive aspect is evident in surface chlorine treatment, which increases the limiting oxygen index from 26 to 36% [37]. Although thermal stability is essential for most applications in terms of the upper temperature limits of materials, it is also very important from a safety perspective to know the products that evolve in a decomposition process. In aromatic polyamides and in halogenated polyamides these products have been studied by pyrolysis methods [38–40].
In general, halogenated groups have been incorporated into polyesters, polyamides, and polyimides, often with the intention of increasing thermal stability or lowering the dielectric constant and surface energy [41, 42].
This work deals with the synthesis and characterization of halogen-substituted polyamides with the aim of fully understanding the effect of halogen substitution on solubility, water uptake, and thermal and mechanical properties; properties that have to be considered to the application of wholly aromatic polyamides in advanced materials, for example, in water desalination membranes and in the aerospace industry. The study of constitutional isomerism and its great influence on chemical microstructures and properties in non-symmetric monohalogen substituted diamines is a further objective of this work.
Experimental section
Materials and methods
Isophthaloyl chloride and terephthaloyl chloride were recrystallized twice from dry heptane. N-methyl-2-pyrrolidone (NMP) was vacuum-distilled twice, firstly over phosphorous pentoxide, and a second time over calcium hydride, and then stored in dark bottles over 4-Å molecular sieves. All other materials were commercially available and were used as received unless otherwise indicated.
Monomers
The synthetic steps, chemical structure and acronyms of all of the monomers are depicted in Scheme 1. 1,3-diamine-4-fluororobenzene, 1,3-diamine-4-chlorobenzene, 1,3-diamine-4-bromobenzene and 1,3-diamine-4,6-difluorobenzene were all synthesized by the same general method. As an illustrative example, the synthesis of 1,3-diamine-4-chlorobenzene is described below.
1,3-diamine-4-chlorobenzene
A mixture of 5.0 g (24 mmol) of 1-chloro-2,4-dinitrobenzene and 8.8 g (74 mmol) of granulated tin was cooled to 0 °C in a 500 ml round-bottomed flask fitted with a mechanical stirrer and a condenser. Then, under vigorous stirring, 40 ml of conc. HCl was added dropwise over a 2 h period. The mixture was allowed to react at 0 °C for 1 h, and then the temperature was increased to 20 °C, and the reaction proceeded for a further 4 h. The solution obtained herein was then heated to 90 °C for 1 h, cooled in an ice bath and 100 ml of dichloromethane was added to the reaction vessel, after which KOH was gradually added to the stirred heterogeneous mixture until a strongly alkaline solution was obtained. The organic layer was washed several times with water, dried with anhydrous sodium sulphate, filtered off, and the dichlorometane removed by rotary vacuum distillation. The dark crude product was purified by double vacuum sublimation at 80 °C and P < 1 mmHg, rendering colourless needles.
1,3-diamine-4,6-dichorobenzene
A mixture of 40 ml (44 mmol) of dry triethylamine, 20 g (185 mmol) of m-phenylenediamine and 200 ml of dichloromethane was cooled to 0 °C in a 500 ml round-bottomed flask fitted with a mechanical stirrer and a condenser. Then, under vigorous stirring, a solution of 37 ml (444 mmol) of acetyl chloride in 75 ml of dichloromethane was added dropwise with an addition funnel over a 3 h period. The mixture was allowed to react at 0 °C for 1 h, and then a solution of KOH/H2O (50/50) was added dropwise until a strong basic solution was obtained. A needle-like brown precipitate was immediately observed. The product, 1,3-diacetamidobenzene, was filtered off, washed thoroughly with water and dried at 70 °C until its weight remained constant. Yield: 21.3 g (60%). The crude product was employed in the following synthetic steps.
A 500 ml round-bottomed flask fitted with a mechanical stirrer and a condenser was filled with a solution of 6.3 g (33 mmol) of 1,3-diacetamidobenzene in 100 ml of anhydrous acetic acid. Chlorine, dry and free of HCl, was bubbled into the former stirred solution, and a white precipitate was immediately obtained. The Cl2 addition was discontinued when no further precipitate formation was visible. The crude 1,3-diacetamide-4,6-dichorobenzene was filtered off, washed thoroughly with distilled water and re-crystallized in ethanol.
1,3-diacetamide-4,6-chorobenzene [2.2 g (11,4 mmol)] was refluxed in a mixture of 40 ml of ethanol and 20 ml of conc. HCl for 4 h. The mixture was cooled slowly to room temperature, the solvent was vacuum distilled, and the 1,3-diamino-4,6-dichorobenzene dihidrochloride obtained herein was solved in 100 ml of distilled water. Upon neutralization with Na2CO3, 1,3-diamine-4,6-dichorobenzene was obtained as a yellowish precipitate, which was then purified by silica gel column chromatography (ethyl acetate/hexane 1:1).
1,3-diamine-4,6-bromobenzene
The former synthetic procedure was followed, but Br2 (liquid) was added dropwise instead of by bubbling Cl2 (gas) in the synthesis of 1,3-diacetamide-4,6-bromobenzene.
Elemental analyses, melting points and yields of all diamines are summarized in Table 1.
Polymer syntheses
A typical polymerization reaction is described: A 100 ml double-walled glass flask, equipped with a nitrogen inlet and a mechanical stirrer was charged with 10.0 mmol of diamine and 20 ml of NMP under a nitrogen blanket. When the diamine was dissolved, 10.0 mmol of trimethylsilyl chloride (TMSCl) was added, and the solution was stirred and cooled to 0 °C by a circulating cooling system. Then, 10.0 mmol of diacid chloride was added portionwise for 5 min, and the mixture was allowed to react under nitrogen for 1 h at 5 °C, and then at 20 °C for a further 3 h. The final very viscous solution was poured slowly onto 800 ml of distilled water, forming a white, fibrous, swollen polymer precipitate, which was filtered off, washed thoroughly with water, extracted with acetone for 24 h in a Soxhlet, and dried in a vacuum oven at 70 °C overnight. The yields were nearly quantitative for all of the polymerizations.
Measurements
1H and 13C NMR spectra were recorded on a Varian INOVA 400 spectrometer operating at 399.92 MHz (1H) and 100.57 MHz (13C), using deuterated dimethylsulphoxide as a solvent and TMS as the internal standard. Microanalyses were carried out by the Analysis Service (SCAI) of Burgos University. Differential scanning calorimetry (DSC) analyses were performed on a Perkin-Elmer Pyris I calorimeter at a heating rate of 20 °C/min under nitrogen. The glass transition temperature (Tg) was taken as the midpoint of the inflection observed on the heat capacity vs. temperature curve. Thermal gravimetric analyses (TGA) were performed on a Perkin-Elmer TGA7 thermobalance. Measurements were recorded with 2 ± 0.5 mg sample heated under a controlled flux of nitrogen at 10 °C/min. Inherent viscosities were determined at 25.0 ± 0.1 °C with and Ubbelohde viscometer using H2SO4 or DMA (5% LiCl) as a solvent, on polymer solutions with a concentration of 0.5 g/dL. Qualitative solubility was determined using 20 mg of polymer in 1 ml of solvent. Water absorption rates were determined by placing samples of about 200 mg of the polyamide in a thermostated circulating air box at 20 °C. 65% r.h. was maintained by means of an oversaturated solution of NaNO2 in water, and 100% r.h. by a water saturated atmosphere. The samples had previously been dried at 100 °C under vacuum for 24 h.
Results and discussion
Synthesis
The mono or dihalogenated diamine monomers were synthesized according to Scheme 1 in high yields and inexpensive routes from commercially available 1,3-dinitro-4-halobenzene, 1,3-dinitro-4,6-difluorobenzene or m-phenylenediamine.
Polymerization was carried out at a low temperature by reaction of stoichiometric amounts of diamines with isophthaloyl or terephthaloyl chloride in an NMP solvent, with TMSCl as a direct activating agent of the amine group (in situ silylation) [43, 44]. Table 2 shows the acronyms and chemical structure of all the polymers.
The values of inherent viscosities (η inh) are listed in Table 3. Inherent viscosities were high enough to assure high molecular weights and developing properties, almost to their full extent, so that as a group they offered a good opportunity for a comparative study.
Characterization and constitutional order
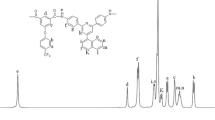
The polyamides were characterized by elemental analysis (Table 2) and by 1H and 13C NMR spectroscopy (as an illustrative example, the spectra of polyamide 2FI are depicted in Fig. 1).
The diamine-containing polymers derived from monohalogen exhibit constitutional isomerism owing to the head-to-tail, head-to-head, and tail-to-tail sequence distribution in the polymer chain (Fig. 2) [30]. This influences the cohesive energy of the polyamides and, as a consequence, other properties such as T g, crystallinity, solubility and mechanical properties. The constitutional order was determined by studying the constitutional regularity (s), which is the probability of two adjacent non-symmetrical units in a chain pointing out in the same direction (head to tail orientation). This study was accomplished with 1H and 13C NMR spectroscopy, evaluating the peaks of the amide groups. Figure 3 shows the peak distribution of 13C NMR associated with the different amide groups of polymers 1FI, 1ClI and 1BrI arising from sequence distribution. Applying our procedure [30], a constitutional order between 0.36 and 0.44 was obtained, in terms of s (Table 4). A value of s = 1 and s = 0 implies a completely ordered structure with a head-to-tail and head-to-head/tail-to-tail arrangement, respectively. The random orientation of sequences, thus the higher disorder, is obtained for a value of s = 0.5.
When the volume of the halogen is increased (from F to Br) and the monomers are compared, a higher polymer constitutional order is observed under common polymerization conditions, implying that the higher volume of the halogen increases the difference in reactivity of the two amine groups in the diamine monomer.
Thermal properties
The thermal properties of the polymers were determined by DSC and TGA.
Glass transition temperatures, as determined by DSC, were high and ranged from 255 to 313 °C (Table 3). This result is significant as the reference technical, multipurpose aramide-like Nomex® [poly(m-phenylene isophthalamide)] shows a T g of 275 °C. It means that modification of the non-substituted fully aromatic polyamide by means of introducing halogen in the amine moiety of the polyamide does not exert any significant influence on T g (in the polyisophthalamides the values of T gs ranged between 255 and 274 °C). A few trends are observed:
-
(a)
Polyterephthalamides have higher T gs than the corresponding polyisophthalamides due to the higher cohesive energies associated with the higher linearity imposed by the 1,4-diphenyl substitution, instead of the 1,3-disubstitution of the diacid moiety.
-
(b)
In most of the polymers, the influence in mono or dihalogen substitution is practically negligible. Taking into account the constitutional disorder of polyamides with only one halogen per structural unit, if perfectly ordered polymers had been obtained, the T gs of those polymers would have been higher than the corresponding polyamides with two halogens. We think that the intrachain interaction between halogens and amide groups diminishes the global hydrogen bond density lowering, to some extent, the T gs.
-
(c)
The higher the halogen Van der Waals volumes (F = 13.37 Å3, Cl = 23.34 Å3, Br = 31.20 Å3), the higher the Tg for each polymer series (Fig. 4).
In any event, the interpretation of T g variations due to chemical substitutions in the main polymer chains is generally not easy to rationalize because of multiple factors: one view might be that increased volumes should impair chain rotation and increase the T g. Conversely, the increase in volume might increase the distance between chains and this should diminish the interchain interactions lowering the T g. Furthermore, if the chemical substitution introduces polar groups, such as halogens, new polar interactions are created, that could increase T g. Halogen, and particularly fluoride, establishes fluoride bonds with, for example, H of amide groups that may exert a double influence on a polyamide: if the bond is interchain, the T g should increase, but if it is intrachain, this should weaken the interchain hydrogen interamide bonds, and lower the T g.
Figure 5 depicts the DSC of polymers 1BrT and 2BrT. After T g, an exothermic crystallization peak is observed, but this is not a clean process because it is accompanied by a chemical dehydrohalogenation process, which leads to a partial polybenzoxazol structure (Scheme 2) [45]. As the hypothetical T m of the polymers is above this chemical reaction, the thermal transition associated with the polymer melt can unfortunately not be studied.
The high decomposition temperatures (T d), ranging between 390 and 430 °C, in terms of TGA onset, correspond to thermally stable polymers, and are shown in Table 3. Another interesting parameter in thermal polymer decomposition is the char yield after decomposition. In these polyamides, the extremely high char yield from 55 to 81% is noteworthy. Decomposition starts with the loss of one or two HX molecules to produce a thermally stable phenylbenzoxazol subunit (Scheme 2), so the char yield at 650 °C is outstandingly higher than the NOMEX® (50%) [46]. In polyamides with two halogens in the structural unit, the formation of a polybenzoxazol structure practically inhibits further degradation in N2 atmosphere, and so, the char yield of these polymers virtually corresponds to the weight loss or two molecules of HF, HCl, or HBr.
All the polymers show a unimodal decomposition behaviour that strongly indicates that decomposition takes place only with the loss of HX molecules, or that the loss of HX molecules is concomitant with the loss of other low molecular weight fragments. Nevertheless, in contrast, Khanna [38] has described bimodal decomposition behaviour in a series of halogenated polyamides.
Water absorption
The ability to absorb water is an important characteristic of polyamides that is mainly related to the polar amide groups. Isothermal sorption of water at 65 and 100% r.h. was measured and the values were related to the polyamide structure. Figure 6 depicts the absorption isotherms of polymers 2XT (X = F, Cl, Br), and Table 5 shows the data obtained as water absorption percentage, molecules of water per structural unit and molecules of water per amide group.
The absorption of water at 65% r.h. is between 2.2 and 8.1%. The water uptake per structural unit ranged between 0.5 and 1.2, which corresponds to an uptake of 0.2 and 0.6 molecules of water per amide groups. In the series of polyamides with one halogen per structural unit, a slight increase in water uptake is observed in polyterephthalamides in comparison with polyisophthalamides. In the series of polyisophthalamides or polyterephthalamides with one or two halogens per repeating unit, water uptake increases with the increasing volume of the halogen. These values hardly reflect the ideal water to amide group interaction in discrete molecules, such as N-propylpropanamide, which is 1.5:1 (water: amide group) [47], but they are close to the uptake of amide groups present in an amorphous main polymer backbone, which ranges between 0.5 and 0.6 water to amide ratio [48, 49]. The water absorption of NOMEX® at 65% r.h. is 0.6 molecules of water per amide group. Thus, the introduction of halogen moieties in the polymeric structural unit diminishes the percentage of water uptake of the halogenated polyamides and, in most cases, also lowers the absorption per amide group.
When water uptake is studied at 100% r.h., the values are virtually double those obtained at 65% r.h.
Solubility
The solubility of the polyamides is shown in Table 6. In general, the highly ordered polyamides derived from symmetric diamines, with two halogens in their structure, are very insoluble, even in organic aprotic solvents with dissolved salts. For example, 2ClT is only soluble in conc. sulphuric acid.
Polyamides with only one halogen per structural unit are soluble in common polar aprotic solvents, such as NMP, except for 1FT that is insoluble in any solvent, and salts as LiCl has to be added to the solvents to promote solubility. This generally higher solubility arises from the asymmetry of the monomers that leads to constitutional isomerism, which diminishes the interactions between interchain amide groups and halogen-amide groups.
The general trends in solubility are depicted in Table 7.
Mechanical properties
Those polyamides that could be dissolved showed a good film-forming ability and could be transformed into films by casting. The mechanical properties of the polyamides are shown in Table 6. They can be considered good for films made on a laboratory scale, with values of 84–145 MPa tensile strength and 2,700 MPa to 3,700 MPa Young’s modulus.
Conclusions
Twelve aromatic polyamides containing one or two halogens (F, Cl and Br) per structural unit have been prepared and characterized. The polymers show high T gs and an outstanding thermal stability in terms of T d and mainly in char yield, which are recognised as properties of the halogenated polymers. The polymers derived from dihalogen substituted diamines show an extremely low solubility due to the molecular symmetry and intramolecular interactions between halogens and amide groups that lead to a high cohesive energy. The polymers with a monohalogen substituted structural unit show constitutional isomerism that promotes solubility and influence the thermal properties of the materials due to the inherent disorder of the head-to-head, head-to-tail and tail-to-tail sequence distribution. Their water uptake is diminished by the introduction of halogen in the poly(m-phenylene isophthalamide) or poly(p-phenylene terephthalamide) structure, and in terms of experimental fully aromatic polyamides, the films derived from these polymers exhibit good mechanical properties.
References
Preston J (1988) In: Mark HF, Bikales NM, Overberger CC, Menges G (eds) Encyclopedia of polymer science and engineering, vol 11. Wiley-Interscience, New York, p 381
Flood JE, White JL, Fellers JF (1982 J Appl Polym Sci 27:2965
Saifrasun S, Amornsakchai T, Sirisinha C, Meesiri W, Bualek-Limcharoen S (1999) Polymer 40:6437
Imai Y (1995) High Perform Polym 7:337
de Abajo J, de la Campa JG, Lozano AE, Alvarez JC (1995) Adv Mater 7:148
Hsiao SH, Yang CP, Chen CW, Liou GS (2005) J Polym Res 12:289
Liou GS, Fang YK, Yen HJ (2007) J Polym Res 14:147
Calderón V, Scwarz G, García F, Tapia MJ, Valente AJM, Burrows HD, García JM (2006) J Polym Sci A Polym Chem 44:6252
Calderón V, García F, de la Peña JL, Maya EM, Lozano AE, de la Campa JG, de Abajo J, García JM (2006) J Polym Sci A Polym Chem 44:4063
Calderón V, García FC, de la Peña JL, Maya EM, García JM (2006) J Polym Sci A Polym Chem 44:2270
García JM, de la Campa JG, Schwarz G, de Abajo J (2001) Macromol Chem Phys 202:1298
García JM, García F, Sanz R, de la Campa JG, Lozano AE, de Abajo J (2001) J Polym Sci Polym Chem 39:1825
García JM, Alvarez JC, de la Campa JG, de Abajo J (1997) Macromol Chem Phys 198:727
Ayala V, Maya EM, García JM, de la Campa JG, Lozano AE (2005) J Polym Sci A Polym Chem 43:112
Gaudiana RA, Minns RA, Rogers HG, Sinta R, Kalganarama P, McGrown C (1987) J Polym Sci Polym Chem Ed 25:1249
Nakata S, Brisson J (1997) J Polym Sci Part A 35:2379
Lin J, Sherrington DC (1994) Adv Polym Sci 111:177
Staubli A, Mathiowitz E, Langer R (1991) Macromolecules 24:2291
Ueda M, Mirishima M, Kakuta M, Sugiyama J (1992) Macromolecules 24:6580
Meyer WR, Gentile FT, Suter UW (1991) Macromolecules 24:642
Li L, Haba O, Endo T, Ueda M (2001) High Perform Polym 13:S217
Ueda M, Sugiyama H (1994) Macromolecules 27:240
Odian G (1991) Principles of polymerization, 3rd edn. Wiley-Interscience, New York, pp 59–66
Gentile FT, Suter UW (1991) Makromol Chem 192:633
Gentile FT, Meyer WR, Suter UW (1991) Macromolecules 24:633
Gentile FT, Suter UW (1989) In: Allens F, Bevington JC (eds) Comprehensive Polymer Science, vol 5. Pergamon, Oxford, p 97
Suter UW, Pino P (1984) Macromolecules 17:2248
Ravindranath K (1990) Polymer 31:2178
Ueda M (1999) Prog Polym Sci 24:699
García JM, García FC, Serna F (2003) J Polym Sci A Polym Chem 41:1202
Orton KJP, Soper FG, Williams G (1928) J Chem Soc 998
Konogaya S, Watanabe O (2000) J Appl Polym Sci 76:201
Van Krevelen DW (1975) Polymer 16:615
Pearce EM, Khanna YP, Raucher D (1981) In: Turi EA (ed) Thermal characterization of polymeric materials, chap 8. Academic, New York
Whang WT, Kapuscinska M, Pearce EM (1986) J Polym Sci Polym Symp 74:109
Kakimoto MA, Yoneyama M, Imai Y (2000) J Polym Sci A Polym Chem 38:3911
Ellis B (ed) (2000) Polymers – a property database. CRC, Boca Raton, FL
Khanna YP, Pearce EM, Smith JS, Burkitt DT, Njuguna H, Hindenlang DM, Forman BD (1981) J Poly Sci Polym Chem Ed 19:2817
Brown JR, Power AJ (1982) Polym Degrad Stab 4:379
Czégény Zs, Blazsó M (2001) J Anal Appl Pyrolysis 58–59:95
Feiring AE (1994) In: Bamks RE, Smart BE, Tatlow JC (eds) Organofluorine chemistry. Principles and commercial applications, chap 15. Plenum, New York
Álvarez JC, García JM, de la Campa JG, de Abajo J (1997) Macromol Chem Phys 198:3293
Oishi Y, Kakimoto M, Imai Y (1987) Macromolecules 20:703
Lozano AE, de Abajo J, de la Campa JG (1997) Macromolecules 30:2507
Kapuscinski M, Pearce EM (1984) J Polym Sci Polym Chem Ed 22:3989
Karydas AC, Whang WT, Pearce EM (1984) J Polym Sci Polym Chem Ed 22:847
Ling GN (1972) In: Horne RA (ed) Water and aqueous solutions. Wiley-Interscience, New York
Koros WJ (1985) J Polym Sci A Polym Chem 23:1611
Sumimoto H, Hashimoto K (1985) Adv Polym Sci 64:63
Acknowledgments
The financial support provided by the Ministerio de Educación y Ciencia (MAT2005-01355) and the Junta de Castilla y León (BU003A05) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Serna, F., García, F., de la Peña, J.L. et al. Properties, characterization and preparation of halogenated aromatic polyamides. J Polym Res 14, 341–350 (2007). https://doi.org/10.1007/s10965-007-9112-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-007-9112-z