Abstract
The more efficient and hydrophobic modificated ammonium polyphosphate (M-APP) and charring agent (M-CA) were prepared based on sol–gel method, and they were used in the preparation of water-resistant and flame-retardant polypropylene (PP) composites. The results showed that the contact angles of M-APP and M-CA were 135.11° and 137.89°, respectively. The flame-retardant and thermal properties of the PP composites were also studied. The results showed that the flame retardancy of PP loaded with 30 mass% intumescent flame retardant (M-APP/M-CA = 3:1) was significantly increased. The limiting oxygen index (LOI) reached 36.8% and passed UL-94 V-0. The peak heat release rate and total heat release of the PP composite decreased from 1011.77 to 134.43 kW·m−2 and 100.58 to 6.86 MJ·m−2, respectively. The peak smoke production rate and total smoke release decreased from 0.13 to 0.0056 m2·s−1 and 1453.09 to 74.82 m2·m−2. The PP composite with M-APP/M-CA = 3:1 also exhibited excellent water resistance, and it could still reach UL-94 V-0 after soaking in 70 ℃ water for 168 h.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polypropylene (PP) is an important thermoplastic material, which is widely used in the fields of electrical equipment housing, vehicle transportation, furniture decoration and so on [1,2,3]. However, PP is easily combustible and releases a large amount of smoke and toxic gases at high temperatures, which severely limits its application [4, 5]. Halogen-containing flame retardants have been widely used in flame retardancy for PP. However, the halogens are harmful to the environment and human healthy [6]. Due to the advantages of eco-friendly and high efficiency, intumescent flame retardants (IFRs) have got more and more attention and shown a good prospect in the flame-retardant application field [7, 8]. Generally, IFRs are composed of “three sources”: acid source, carbon source and gas source [9]. Ammonium polyphosphate (APP) is a widely used acid source and shows the excellent property of flame retardancy and thermal stability. For carbon source, the agents containing triazine ring or triazine-like ring have attracted the attention of researchers. For example, Liu et al. prepared flame-retardant PP composites by blending carbon forming agent triazine (CFA) [10]. Chen et al. used tri-(2-hydroxyethyl) isocyanurate as charring agent to prepare an intumescent flame retardant for PP [11]. Although a lot of researchers have studied and proved the high efficiency of IFRs, there still exist some defects such as the relatively low water resistance [12, 13].
The microencapsulation is one of the most important methods to solve water resistance problem. For example, Ding et al. synthesized a novel microencapsulated APP (MAPP) with cross-linked β-cyclodextrin. The contact angle test results demonstrated that the hydrophobicity of microencapsulating MAPP was improved significantly [14]. Li et al. prepared a new type of microcapsule flame-retardant aluminum hypophosphite microcapsule and used it to prepare flame-retardant EP composites. The results showed that the contact angle of the microcapsule flame retardant was increased from 34.4° to 71.4° after modification [15]. In this context, to achieve higher flame-retardant efficiency while ensuring the excellent water resistance of the matrix has become an important target for researchers when designing and developing IFRs system.
In this work, the more efficient and hydrophobic modificated ammonium polyphosphate (M-APP) and charring agent (M-CA) were prepared based on sol–gel method, and they were used in the preparation of water-resistant and flame-retardant PP composites. The flame-retardant and thermal properties of the PP composites were studied by limiting oxygen index (LOI), UL-94, cone calorimetry test (CCT), water immersion test, thermogravimetric–Fourier transform infrared spectroscopy (TG–IR) and X-ray photoelectron spectroscopy (XPS). From this work, we hope to provide a method to further improve the water resistance of flame-retardant PP composites.
Experimental
Materials
PP (F401) was bought as pellets from Yangzi Petrochemical Co., Ltd. (Huainan, China). Ammonium polyphosphate (APP, phase II, polymerization degree > 1000) was brought from Shandong Shian Chemical Co., Ltd. (Shandong, China). CA was purchased from Heilongjiang Orient Science and Technology Co., Ltd (Heilongjiang, China). Tetraethoxysilane/triethyl orthosilicate (TEOS, CP), alkylphenol polyoxyethylene (OP-10, AR), ammonia solution (25 mass%, AR) and ethanol (99.7 mass%, AR) were purchased from Sinopharm Chemical Reagent Co., Ltd. Vinyltrimethoxysilane (A-171, Tech) was supplied by Nanjing Quanxi Chemical Co., Ltd (Nanjing, China). All materials were utilized as received without further purification in this study.
Synthesis of M-APP (or M-CA)
The ethanol (150 mL) and the deionized water (50 mL) were added into a 500-mL three-neck round-bottom flask equipped with a reflux condenser and a mechanical stirrer; then, the mixture was stirred and heated up to 45 ℃. After that, APP (50 g), OP-10 (1 g) and ammonia (20 mL, 25 mass%) were introduced into the flask, keeping stirring for 10 min. Then, 10 g TEOS was added by dropwise over a period of 30 min and kept the reaction for 4 h. Finally, A-171 (2.5 g) was added into the flask and further reacted at 60 ℃ for 1 h. After the reaction was completed, the mixture was cooled to the room temperature, filtered, washed with deionized water and dried at 80 ℃ until the mass remained unchanged. The yield of M-APP and M-CA was about 83, 84%, respectively.
Fabrication of the PP composites
Materials were dried in a vacuum oven at 80 ℃ for 12 h before use in this experiment. After then, PP, CA, M-CA, APP and M-APP were mixed evenly according to the formulation listed in Table 1. Pure PP pellets and uniformly mixed flame-retardant powder were simultaneously slowly added into a torque rheometer (XSS-300, Shanghai Kechuang Plastic Machinery Co., Ltd., China) over a period of 8 min at 170 ℃ with a rotor speed of 60 rpm. Then, the composites were melted blending for another 10 min at the same temperature. Subsequently, the composites were hot-pressed into sheets under the conditions of 10 MPa and 170 °C for 5 min by an electric vulcanizing machine (XH-406, Dongguan City Xihua Testing Machines Co., Ltd. China). The detail formulations for samples in this article are shown in Table 1.
Characterization
The limited oxygen index (LOI) texts were measured using a HC-2 oxygen index meter (Jiangning Analysis Instrument Co., China). The dimension of specimens which used for the LOI test was 100 × 6.5 × 3 mm3. The vertical burning test was evaluated by using a CZF-5 vertical burning tester (Jiangning Analysis Instrument Company, China) with the dimension of sheets was 100 × 13 × 3 mm3. LOI tests and UL-94 tests were carried out on the basis of the standard of oxygen index test ASTM D2863 and UL-94, respectively.
Thermal gravimetric analysis (TGA) curves were performed with SDTA851e TA instrument (Merit Toledo Co., Ltd., Switzerland). The samples were heated from 30 to 700 ℃ at a heating rate of 10 ℃ min−1. About 5–10 mg samples were added into an open Pt pan and heated from 30 to 800 ℃ in air atmosphere at a heating rate of 10 ℃ min−1.
Cone calorimeter (CCT, TESTech Instrument Technologies Co., Ltd., China) was used to evaluate the flammability characterization with a heat flux of 50 kW·m−2 which was based on the standard of ASTM E1354. The dimension of specimens used for the CCT test was 100 × 100 × 3 mm3.
Thermogravimetric–Fourier transform infrared spectroscopy (TG–FTIR) curves were obtained by TGA5500 thermogravimetric analyzer (TA, USA) coupled with IS50 FTIR spectrophotometer (Nicolet, USA) from 200 to 800 °C with a linear heating rate of 20 °C min−1 under the air.
The mechanical tests of PP composites were measured with a universal testing machine (WDW-20, Ji’nan Xinshijin Testing Machine Co., Ltd.). PP composites samples have a dumbbell-like shape with the size of 25 × 6 × 1 mm3 (effective section).
XPS was measured with the ESCFALAB 250Xi electron spectrometer (Thermo Fisher Scientific Co., America). The Al Kα radiation (1486.68 eV) was utilized as the excitation source.
In the scanning electron microscopy (SEM) text, all samples were sprayed with gold before measurement. The morphological structure of flame retardants and the char residue of the composites, which was obtained from the CCT text, were studied by using Gemini SEM 500 (ZEISS Co., Germany) and kyky-2800B SEM (KYKY Co., China), respectively. The element distribution was tested by an energy-dispersive spectroscopy (EDS, Hitachi S-4800 and JEM-2100, Japan).
The contact angle was evaluated by a drop-shape analysis system (POWERREACH, Model JC2000A). The powder of CA, M-CA, APP and M-APP was homogeneously dispersed on a slide, whose surface was pressed by using another slide to get flat.
Results and discussion
Characterization of M-CA and M-APP
The FTIR spectra of APP, M-APP, CA and M-CA are shown in Fig. 1. APP contains the characteristic group of N–H and P–O, and characteristic peaks can be observed at 3162 cm−1 and 916 cm−1, respectively. The asymmetric stretching and symmetric stretching vibration of P=O are observed at 1678 and 1275 cm−1, respectively [16]. After being microencapsulated, M-APP shows new absorption bands at 1106 and 806 cm−1, which is, respectively, attributed to the absorption of Si–O–Si and Si–OH, demonstrating a successful surface modification [17]. The same situation also occurs in the spectrum of CA in Fig. 1b. The typical absorption peaks of CA include 1500 (–N=C–) cm−1 and 1546 (N–H) cm−1[18]. After modification, M-CA presents the new absorption peaks of C=C (1645 cm−1) and Si–O–Si (1093 cm−1), which means a successful surface reaction [16].
In order to further confirm the modification, the morphology of the APP, M-APP, CA and M-CA is observed using SEM (Fig. 2). From Fig. 2a and c, it can be found that pure APP and CA present a smooth outside surface. After being microencapsulated, the surfaces of both APP and CA change obviously and become very rough. There are many silica spheres on it, which result from the hydrolysis of silane [19]. The changes of the morphological structure for the M-APP and M-CA further confirm that the microencapsulation has been successfully implemented. In addition, element mapping images are used to further analyze the elements of M-APP and M-CA. From Fig. 3, the EDS mapping images reveal the presence of P, N and Si in M-APP and also confirm the presence of C, N and Si in M-CA.
The water resistance of M-APP and M-CA
The water resistance of M-APP and M-CA is further evaluated by static water contact angle measurements (Fig. 4). The experimental results show that APP and CA materials are easily soaked by water droplets, because they are composed of polar units, leading to the super hydrophobic nature [10]. Therefore, the corresponding water contact angle of APP and CA is 0°. Interestingly, the water contact angle values of M-APP and M-CA are 135.11° and 137.89°, respectively. Furthermore, the water drops maintain a stable shape on the M-APP and M-CA surfaces for a long time. The low polarity of the modifier, which contains the hydrophobic groups on the outside surface, leads to an efficient incompatibility with the polar bonds in H2O. In addition, numbers of the spherical particles distributing on the surfaces of M-APP and M-CA form the micro-nano structure like the lotus leaf, which further improves the water resistance property [20, 21].
The water resistance of M-APP and M-CA is further confirmed by water solubility test. And the test results show that the water solubility of APP and CA is 1.026 g/100 mL and 0.1050 g/100 mL at 25 ℃, respectively. At the same temperature, the water solubility of M-APP and M-CA decreases to 0.2600 g/100 mL and 0.006 g/100 mL after modification, respectively, which decreased by 74.7% and 94.3%. Those results also show that the water resistance of modifying M-APP and M-CA is significantly improved.
Thermal stability of M-CA and M-APP
The TG and DTG curves are shown in Fig. 5. As shown in Fig. 5a and b, the first decomposition temperature for pure APP is 325 ℃, which is attributed to the release of volatile products such as NH3 and H2O. The second decomposition appears at 495 ℃, which may be caused by the APP evaporates and some dehydrates to generate P4O10 [22]. Compared with pure APP, it can be clearly observed that the yield of residual char which belongs M-APP (32.3 mass%) is higher than that of pure APP (27.3 mass%) at the high temperature. From Fig. 5a, it can also be observed that the mass residue of M-CA (8.5 mass%) is higher than that of pure CA. In addition, it can be seen from Fig. 5b that the maximum mass loss rate of modified M-CA decreases from 0.66 to 0.33%·℃−1. Those results of TG and DTG demonstrate that the method of silicon microencapsulated can enhance the thermal stability of APP and CA, especially in high temperature.
Flammability of the PP composites
UL-94 and LOI tests were used to investigate the flame retardancy of composites. The results of UL-94 and LOI are listed in Table 1. The combustion process of selected composite is shown in Fig. 6. As shown in Fig. 6 and Table 1, the pure PP burns intensely in the air with severe melt dripping. Its LOI value is only 18.7 ± 0.2%. PP1 and PP2 both contain single flame retardancy. PP1 containing 30 mass% M-CA burns rapidly and completely after ignited, and the resulting melt droplets ignite the cotton below. The results show that PP1 has no rate in UL-94 test, and the LOI value is just 19.6 ± 0.2%. The PP2 containing 30 mass% M-APP also burns rapidly and completely; the melt drips generated ignite the cotton below. The results show that PP2 has no rate in UL-94 test and its LOI value is 22.7 ± 0.2%. The flame-retardant PP composites (PP3, PP4 and PP5) with a series ratio of M-APP to M-CA, from the 2 to 4, are prepared. The results show that all composites reach a V-0 rate. Among of them, PP4 in which the ratio of M-APP and M-CA is 3:1 shows the optimal flame retardancy, and its LOI value is as high as 36.8 ± 0.2%. Meanwhile, it can be seen from Fig. 6 that during the UL-94 test, PP4 extinguishes rapidly after the first ignition and extinguishes automatically in 5 s after the second ignition. The series results demonstrate that M-APP and M-CA present a positive synergistic effect and give the PP composites great improvement in flame retardancy. It is worth to mention that when the ratio of M-APP and M-CA is 3:1, it endows the PP composite with the highest flame-retardant efficiency.
The water-resistant test for PP composites
The water resistance of the flame-retardant PP composites is further investigated. The PP4 with the best flame retardancy is chosen and dipped it into 70 ℃ water for different periods of time. The results of UL-94 test are listed in Table 2. After being soaked in 70 ℃ water for 168 h, PP4 still maintains its high efficiency of flame retardant, reached the V-0 rate in UL-94 without any drips.
The mechanical properties of PP composites
The mechanical properties test results of PP and PP4 composites are shown in Table 3. Generally speaking, the increase in additive loading may lead to the deterioration of mechanical properties of polymer matrix, which is mainly due to the poor compatibility of additive matrix. In this study, the tensile strength of pure PP is 39.33 ± 0.8 MPa and the elongation at break is 19.62 ± 0.5%. However, the tensile strength and elongation at break of PP4 composites with 30 mass% intumescent flame retardant are 40.44 ± 0.5 MPa and 4.63 ± 0.1%, respectively. The results show that the addition of 30 mass% flame retardant has little effect on the mechanical properties of PP composites.
The conical calorimetry test for PP composites
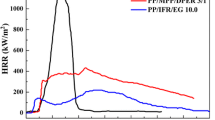
As we all know, the fire risks and fire hazards of composite materials can be evaluated by CCT test, which can record the values of heat release, smoke release, the production of CO and CO2 under the whole combustion process of the composite material from ignition to extinguishment [23, 24]. And the CCT test results of selected composites are shown in Fig. 7.
According to Fig. 7a, it can be seen that pure PP presents a rapid and intense heat release process, in which the peak of heat release rate (pHRR) reached as high as 1011.77 ± 37.42 kW·m−2. When M-APP and M-CA (the ratio of M-APP to M-CA is 3:1) are added together, the pHRR value of PP4 is as low as 134.43 ± 5.83 kW·m−2, which is decreased by 86.71% compared with pure PP. This phenomenon is also observed in total heat release (THR) curves shown in Fig. 7b. The pure PP shows the values of THR are 100.58 ± 1.73 MJ·m−2. As to PP4, it shows the lowest value of 6.86 ± 0.12 MJ·m−2, which is decreased by 93.18% than that of pure PP2.
Smoke release is another important fire behavior which is generally used to evaluate the fire safety of materials. From Fig. 7c and d, one can see that the smoke production rate (SPR) of composites shows similar curves with those in HRR, which demonstrate that the smoke and the heat release are a couple of correlated processes. For pure PP, its peak SPR (pSPR) is 0.13 ± 0.033 m2·s−1, showing a drastic smoke release. The pSPR of PP4 is 0.0056 ± 0.00032 m2·s−1, which is decreased by 95.69% compared with pure PP. The total smoke release (TSR) of PP4 (74.82 ± 1.60 m2·m−2) is also the lowest among the selected composites which is decreased by 94.85% compared with PP (1453.09 ± 30.68 m2·m−2). CO and CO2 are the main gas production of polymers during combustion, and their release is another crucial index which determine the fire safety. From Fig. 7e and f, the peak of CO production value of PP4 is 0.0015 ± 8.4E-05 g·s−1, which is decreased by 83.0% compared with pure PP (0.0088 ± 0.0091 g·s−1). The peak of CO2 production value is also as low as pure PP, which is decreased from 0.56 ± 0.0013 g·s−1 to 0.06 ± 0.0014 g·s−1 with a decrease of 89.3%.
Thermal property of the PP composites
The thermal stability of PP and PP4 is analyzed by TG test. The corresponding data are shown in Fig. 8 and Table 4. And in Table 4, T5%/℃ is the 5% mass loss temperature, Tmax/℃ represents the maximum mass loss rate temperature and the PMLR is the maximum mass loss rate. Furthermore, The Wexp and Wcal represent the experimental and calculated values of residual mass, respectively, and ∆W = Wexp–Wcal[25].
It can be seen from Fig. 8 and Table 4 that for pure PP, the decomposition temperature of T5mass% is 275 °C and the maximum thermal decomposition rate is 1.49%·℃−1 which appears at 360 °C. Above 410 °C, pure PP has completely decomposed without any mass residue. For PP4, the maximum decomposition rate is 0.66%·℃−1 which is decreased by 55.7% than that of pure PP and the residual char of PP4 at 700 °C maintains about 25.13% which is much than that of pure PP. These both exhibit the PP4 has a much higher thermal stability than pure PP in the high temperature. In addition, ∆W can be used to describe this char-forming ability, and the composites with a high ∆W value have a better char-forming ability [26]. As shown in Table 4, the ∆W value of PP4 at 700 ℃ is 14.4 mass%, which indicates that IFRs (30 mass%, M-APP / M-CA = 3:1) can significantly promote the carbonization ability of PP matrix at high temperature. Therefore, the TG and DTG results can adequately illustrate that the combination of M-APP/ M-CA can effectively improve the thermal stability of PP.
TG–FTIR is further conducted to analyze the thermal decomposition process of PP4 in detail. From Fig. 9, it can be observed that all the products start to be detected above 280 ℃, demonstrating that PP4 starts to be decomposed at about this temperature, which coincides with the TG results. Above 280 ℃, the absorption bands at 3400–4000, 2340–2370 and 930–960 cm−1 are attributed to the H2O, CO2 and NH3, respectively, which are the main decomposition products of APP[27]. With the increase in the temperature, the infrared absorption peaks at 2966, 1645, 1458 and 1378 cm−1 are gradually increased. They are attributed to the alkanes, alkenes and diolefins which decompose from PP and show the main process of combustion [28]. As the temperature further increases to 380 °C, new vibration bands began to appear at 1250 and 1030 cm−1. These vibration bands can be attributed to various phosphorus oxides (P–O) which can trap OH or H free radicals in the gas phase to play a role of flame retardant[29, 30].
Analysis of the outer char residues
The char residue which obtained in the analysis of CCT has been reserved after testing, and their outside morphology in conventional and microscale is observed by digital camera and scanning electron microscope (SEM). The photographs are collected and shown in Fig. 10. As shown in Fig. 10a, pure PP burns completely and has no residue char. Then, combining M-APP with M-CA, the amount of char residue formed by PP4 is significantly increased. Figure 10b and d shows the top view and side view of the char residue after combustion, respectively. Compared with the 3 mm thickness of the flame-retardant PP composite, the height of expanded carbon layer formed after CCT test can reach 3.1 cm, and the expansion degree of carbon layer is about 9.3 times. Furthermore, the SEM photograph (Fig. 10c) shows that the char layer exhibits an intumescent and dense outside surface on which a lot of humps distributed. The dense and intumescent char layer plays the role as the physical barrier which hinds the transferring of combustible gas, which makes a great contribution to the improvement of flame retardancy.
To further investigate the solid phase generated during combustion, the XPS is conducted to further analyze the residue char of PP4. The corresponding curves of XPS test are shown in Fig. 11, and the proportion of the constituent elements is shown in Table 5. As can be seen from Fig. 11a and Table 5, the residue char of PP4 after combustion is mainly composed of C, O, N, P and Si, respectively. And the ratios of these elements are 50.5, 31.9, 7.3, 8.9 and 1.4%, respectively. It can be found from Fig. 11b that the three bands at 284.4 eV, 285.7 eV and 286.7 eV are found in the C1s spectra. They can be assigned to C–C bonds and C–H bonds in aliphatic, aromatic structures and graphite and C–O bonds in the P–O–C groups in ether and phosphate, respectively [31, 32]. For O1s, the peaks appear at 531.1, 532.4 and 533.3 eV are mainly attributed to the group of =O– and –O–. The –O– groups may come from the C–O–C, O=P–O–C or O=C–O–P bonds and the =O– groups are probably derived from in –C=O or P=O bonds in carbonyl compounds and phosphate from residue char [31, 33]. As for N1s, there are four peaks appearing at 398.6, 400.0, 401.6 and 405.0 eV. These bands may be ascribed to nitrogen functionalities in pyridinic and in pyrrolic groups, to quaternary nitrogen and to some form of oxidized nitrogen [34, 35]. For P2p, two strong fitting peaks appear in the P2p spectrum, where the peak at 133.5 eV is ascribed to P–N bond and the other peak at 134.1 eV has corresponded to P–O bond [34, 36]. In Si2p XPS spectrum displayed in Fig. 11f, the three peaks at 103.85, 103.05 and 101.40 eV correspond to Si–O–Si, Si–O–P and Si–O–C, respectively[37]. The formation of the cross-linked P–O–C and Si–O–P groups is helpful to form dense and coherent char layers, improving the flame retardancy of PP[38].
Conclusions
A more efficient and hydrophobicity dehydrant (M-APP) and charring agent (M-CA) were successfully prepared based on sol–gel method, and the M-APP and M-CA presented outstanding hydrophobicity and water resistance. The water contact angle test results show that the contact angle of M-CA and M-APP is 135.11° and 137.89°, respectively. The results showed that the flame retardancy of PP loaded with 30 mass% intumescent flame retardant (M-APP/M-CA = 3:1) was significantly increased. The limiting oxygen index (LOI) reached 36.8% and passed UL-94 V-0. The peak heat release rate and total heat release of the PP composite decreased from 1011.77 to 134.43 kW·m−2 and 100.58 to 6.86 MJ·m−2, respectively. The peak smoke production rate and total smoke release decreased from 0.13 to 0.0056 m2·s−1 and 1453.09 to 74.82 m2·m−2. The PP composite with M-APP/M-CA = 3:1 also exhibited excellent water resistance, and it could still reach UL-94 V-0 after soaking in 70 ℃ water for 168 h. TG–IR test results show that there are some phosphorus oxides (P–O) which play a role of flame retardant and can trap OH or H free radicals in the gas phase. Furthermore, the SEM and XPS tests present that the char layers with cross-linked P–O–C and Si–O–P groups show an intumescent and dense outside surface which can play the role as the physical barrier to hind the transferring of combustible gas.
Availability of data and materials
Every data and materials exist in this manuscript are available.
References
Chen S, Li J, Zhu Y, Guo Z, Su S. Increasing the efficiency of intumescent flame retardant polypropylene catalyzed by polyoxometalate based ionic liquid. J Mater Chem A. 2013;1(48):15242–6.
Li J, Lai X, Li H, Zeng X, Liu Y, Zeng Y, Jiang C. Functionalized ZrP nanosheet with free-radical quenching capability and its synergism in intumescent flame-retardant polypropylene. Polym Adv Technol. 2019;31(3):602–15.
Sun Y, Yuan B, Shang S, Zhang H, Shi Y, Yu B, Qi C, Dong H, Chen X, Yang X. Surface modification of ammonium polyphosphate by supramolecular assembly for enhancing fire safety properties of polypropylene. Compos Part B-Eng. 2020;181:107588.
Feng C, Liang M, Jiang J, Huang J, Liu H. Synergistic effect of a novel triazine charring agent and ammonium polyphosphate on the flame retardant properties of halogen-free flame retardant polypropylene composites. Thermochim Acta. 2016;627:83–90.
Wang X, Wang Z, Li J. Effects of a semi-bio-based triazine derivative on intumescent flame-retardant polypropylene. Polym Adv Technol. 2019;30(5):1259–68.
Xu S, Li SY, Zhang M, Zeng HY, Wu K, Tian XY, Chen CR, Pan Y. Fabrication of green alginate-based and layered double hydroxides flame retardant for enhancing the fire retardancy properties of polypropylene. Carbohydr Polym. 2020;234:115891.
Kim NK, Dutta S, Bhattacharyya D. A review of flammability of natural fibre reinforced polymeric composites. Compos Sci Technol. 2018;162:64–78.
Xing W, Wang X, Song L, Hu Y. Enhanced thermal stability and flame retardancy of polystyrene by incorporating titanium dioxide nanotubes via radical adsorption effect. Compos Sci Technol. 2016;133:15–22.
Zheng Z, Qiang L, Yang T, Wang B, Cui X, Wang H. Preparation of microencapsulated ammonium polyphosphate with carbon source- and blowing agent-containing shell and its flame retardance in polypropylene. J Polym Res. 2014;21(5):1–15.
Liu J-C, Xu M-J, Lai T, Li B. Effect of surface-modified ammonium polyphosphate with KH550 and silicon resin on the flame retardancy, water resistance, mechanical and thermal properties of intumescent flame retardant polypropylene. Ind Eng Chem Res. 2015;54(40):9733–41.
Chen, W.; Yuan, S.; Sheng, Y.; Liu, G., Effect of charring agent THEIC on flame retardant properties of polypropylene. Journal of Applied Polymer Science 2015, 132 (1).
Shao Z-B, Deng C, Tan Y, Chen M-J, Chen L, Wang Y-Z. Flame retardation of polypropylene via a novel intumescent flame retardant: Ethylenediamine-modified ammonium polyphosphate. Polym Degrad Stab. 2014;106:88–96.
Shi Y, Gui Z, Yuan B, Hu Y, Zheng Y. Flammability of polystyrene/aluminim phosphinate composites containing modified ammonium polyphosphate. J Therm Anal Calorim. 2017;131(2):1067–77.
Ding S, Liu P, Zhang S, Ding Y, Wang F, Gao C, Yang M. Preparation and characterization of cyclodextrin microencapsulated ammonium polyphosphate and its application in flame retardant polypropylene. J Appl Polym Sci. 2020;137(34):49001.
Li Y, Jiang J, Chen Z, Chen Z, Ni L. Preparation and characterization of microencapsulated aluminum hypophosphite and its performance on the thermal, flame retardancy, and mechanical properties of epoxy resin. Polym Compos. 2021;42(4):1818–34.
Zhu J, Lu X, Yang H, Xin Z. Vinyl polysiloxane microencapsulated ammonium polyphosphate and its application in flame retardant polypropylene. J Polym Res. 2018;25(4):1–7.
Yang X, Tu Q, Shen X, Pan M, Jiang C, Lai X, Xue J. Synergistic modification by mercapto hyperbranched polysiloxane and functionalized graphene oxide on the surface of aramid fiber. Polym Test. 2020;91:106783.
Nie S, Hu Y, Song L, He Q, Yang D, Chen H. Synergistic effect between a char forming agent (CFA) and micro encapsulated ammonium polyphosphate on the thermal and flame retardant properties of polypropylene. Polym Adv Technol. 2008;19(8):1077–83.
Kim J-H, Hossain SM, Kang H-J, Park H, Tijing L, Park GW, Suzuki N, Fujishima A, Jun Y-S, Shon HK, Kim G-J. Hydrophilic/hydrophobic silane grafting on TiO2 nanoparticles: photocatalytic paint for atmospheric cleaning. Catal. 2021;11(2):193.
Hu J, Fang Z, Huang Y, Lu J. Fabrication of superhydrophobic surfaces based on fluorosilane and TiO2/SiO2 nanocomposites. Surf Eng. 2020;37(3):271–7.
Tong W, Xiong D, Zhou H. TMES-modified SiO2 matrix non-fluorinated superhydrophobic coating for long-term corrosion resistance of aluminium alloy. Ceram Int. 2020;46(1):1211–5.
Ni J, Chen L, Zhao K, Hu Y, Song L. Preparation of gel-silica/ammonium polyphosphate core-shell flame retardant and properties of polyurethane composites. Polym Adv Technol. 2011;22(12):1824–31.
Tawiah B, Zhou Y, Yuen RKK, Sun J, Fei B. Microporous boron based intumescent macrocycle flame retardant for poly(lactic acid) with excellent UV protection. Chem Eng J. 2020;402:126209.
Wang W, Wang J, Wang X, Wang S, Liu X, Qi P, Li H, Sun J, Tang W, Zhang S, Gu X. Improving flame retardancy and self-cleaning performance of cotton fabric via a coating of in-situ growing layered double hydroxides (LDHs) on polydopamine. Prog Org Coat. 2020;149:105930.
Yan L, Xu Z, Wang X, Deng N, Chu Z. Preparation of a novel mono-component intumescent flame retardant for enhancing the flame retardancy and smoke suppression properties of epoxy resin. J Therm Anal Calorim. 2018;134(3):1505–19.
Si M, Feng J, Hao J, Xu L, Du J. Synergistic flame retardant effects and mechanisms of nano-Sb2O3 in combination with aluminum phosphinate in poly(ethylene terephthalate). Polym Degrad Stab. 2014;100:70–8.
Wu K, Song Y, Lu H, Wang Z. Flame retardancy and thermal degradation of intumescent flame retardant starch-based biodegradable. Compos. 2009;48(6):3150–7.
Zhang F, Zhang J, Sun D. Study on thermal decomposition of intumescent fire-retardant polypropylene by TG/Fourier transform infrared. J Thermoplast Compos Mater. 2009;22(6):681–701.
Dong H, Yuan B, Qi C, Li K, Shang S, Sun Y, Chen G, Zhang H, Chen X. Preparation of piperazine cyanurate by hydrogen-bonding self-assembly reaction and its application in intumescent flame-retardant polypropylene composites. Polym Adv Technol. 2020;31(5):1027–37.
Lai, X.; Yin, C.; Li, H.; Zeng, X., Synergistic Effect Between Silicone-Containing Macromolecular Charring Agent and Ammonium Polyphosphate in Flame Retardant Polypropylene. Journal of Applied Polymer Science 2015, 132 (10).
Feng P, Ma L, Wu G, Li X, Zhao M, Shi L, Wang M, Wang X, Song G. Establishment of multistage gradient modulus intermediate layer between fiber and matrix via designing double “rigid-flexible” structure to improve interfacial and mechanical properties of carbon fiber/resin composites. Compos Sci Technol. 2020;200:108336.
Zheng Z, Zhang L, Liu Y, Wang H. A facile and novel modification method of β-cyclodextrin and its application in intumescent flame-retarding polypropylene with melamine phosphate and expandable graphite. J Polym Res. 2016;23(4):1–17.
Guo D, Wang Q, Bai S. Poly(vinyl alcohol)/melamine phosphate composites prepared through thermal processing: thermal stability and flame retardancy. Polym Adv Technol. 2013;24(3):339–47.
Zheng Z, Liu Y, Dai B, Meng C, Guo Z. Fabrication of cellulose-based halogen-free flame retardant and its synergistic effect with expandable graphite in polypropylene. Carbohydr Polym. 2019;213:257–65.
Bourbigot S, Michel LB, Gengembre L. XPS study of an intumescent coating II. Application to the ammonium polyphosphate/pentaerythritol/ethylenic terpolymer fire retardant system with and without synergistic agent. Appl Surf Sci. 1997;120:15–29.
Zhang M, Ding X, Zhan Y, Wang Y, Wang X. Improving the flame retardancy of poly(lactic acid) using an efficient ternary hybrid flame retardant by dual modification of graphene oxide with phenylphosphinic acid and nano MOFs. J Hazard Mater. 2020;384:121260.
Zhou T, Xu H, Cai L, Wang J. Construction of anti-flame network structures in cotton fabrics with pentaerythritol phosphate urea salt and nano SiO2. Appl Surf Sci. 2020;507:145175.
Yang R, Ma B, Zhang X, Li J. Fire retardance and smoke suppression of polypropylene with a macromolecular intumescent flame retardant containing caged bicyclic phosphate and piperazine. J Appl Polym Sci. 2019;136(25):47593.
Acknowledgements
The authors deeply appreciate the Provincial Natural Science Foundation of Anhui (1908085J20, 2008085QE269), the National Natural Science Foundation of China (No. 51775001), the University Synergy Innovation Program of Anhui Province (GXXT-2019-027, GXXT-2020-079), Post-graduate’s Innovation Fund Project of Anhui University of Science and Technology (NO.2020CX2016), Natural Science Research Project of Universities in Anhui Province (KJ2020A0326) and Leading Talents Project in Colleges and Universities of Anhui Province.
Author information
Authors and Affiliations
Contributions
Wei Yang contributed to experiment, data curation, investigation and writing—original draft preparation. Xiang Dong contributed to visualization and writing—review and editing. Shi-bin Nie contributed to conceptualization, methodology, writing—review and editing and supervision. Ji-nian Yang contributed to conceptualization and methodology. Xiang-feng Zhang contributed to methodology and formal analysis. Xue-qiang Wu carried out measurements and contributed to formal analysis. Cheng-ye Fang carried out measurements. Hai-lin Su contributed to validation.
Corresponding authors
Ethics declarations
Conflicts of interest
No conflict of interest exists in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, W., Dong, X., Nie, Sb. et al. Flame-retardant and thermal properties of highly efficient water-resistant intumescent flame-retardant polypropylene composites. J Therm Anal Calorim 147, 7323–7336 (2022). https://doi.org/10.1007/s10973-021-11038-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-11038-0