Abstract
The contraction of poly(acrylic acid-co-butyl methacrylate) (P(AA-co-BMA)) gel induced by complexation with linear Poly(N-vinyl-2-pyrrolidone) (PVP) is quite different from that of poly(acrylic acid) (PAA) or poly(methacrylic acid) (PMAA) gel. The dynamic mechanic properties vary greatly between complexed and uncomplexed networks. It was found that the concentration of PVP has a strong effect on the complexation with P(AA-co-BMA) gel and the dynamic mechanic properties of the P(AA-co-BMA)/PVP complexes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer gels have attracted much interest because they have the ability to react to changes in the external conditions by considerable volume changes, swelling or shrinkage. The external stimulus includes not only temperature changes, but also a variation in the pH value, the ionic strength, or the quality of the solvent, and so on. Therefore, polymer gels have been explored to perform various functions including artificial organs and muscles [1], bioseparations [2], and supports for biocatalysts [3, 4]. The drastic shrinkage of the gel volume as a result of the interaction between the hydrogel and different compounds is investigated as well. Many polymer-polymer complexation systems with synthetic macromolecules have been investigated in the last decade [5–7]. Interpolymer complexes are formed through some secondary binding forces such as hydrogen bonding, Coulombic force, or hydrophobic interaction in aqueous medium [8, 9].
Poly(N-vinyl-2-pyrrolidone) (PVP) is one of the most frequently investigated classes of materials for use in medicine and in other applications interfacing with biological systems. The principal reason for successful PVP application is their excellent biocompatibility with living tissues and extremely low cytotoxicity. PVP can form stable complexes with polyacids through hydrogen bonding, and this kind of complexes is the most stable of the hydrogen bonded complexes [5, 10–12]. Iliopoulos and Audebert [13] studied the complexation of poly(acrylic acid) (PAA) at varying degrees of ionization with PVP. Liu et al. [10] also studied the complexation between poly(methacrylic acid) (PMAA) and PVP in aqueous phase. Osada showed that the absorption of PVP leads to the contraction of the gel of PMAA or PAA [12, 14]. However, research work concerning the swelling behavior of the complexes between the copolymer network containing hydrophobic monomer and PVP has not been presented previously because of the complexity of such systems. Meanwhile, there are no reports on the dynamic mechanic properties of the complexes between the polymer network and PVP. In the present study the complexes of poly(acrylic acid-co-butyl methacrylate) (P(AA-co-BMA)) network with PVP stabilized by the hydrogen bonding were prepared. As comonomer with AA, butyl methacrylate (BMA) has been selected due to its hydrophobic character. By introducing the PBMA component, a large contraction of gel induced by complexation with PVP can be averted, which makes it possible to obtain the results of the mechanical properties of complexes. In this paper, the conformational changes of P(AA-co-BMA) gel induced by PVP were examined, and the dynamic mechanical properties of P(AA-co-BMA)/PVP were studied.
Experimental
Materials
Acrylic acid (AA), butyl methacrylate (BMA), 2,2′-azobis(isobutyronitrile) (AIBN) and N,N′-methylenebis(acrylamide) (MBAA) were analytical grade from Chengdu Reagent Factory. Samples of poly(N-vinyl-2-pyrrolidone) (PVP) with number-average molecular weights of 30,000 (Aldrich) were used as received. AA and BMA were distilled under reduced pressure before use. AIBN, used as a radical initiator, was recrystallized from ethanol solution. MBAA, used as a cross-linker, and PVP were used without further purification.
Preparation
P(AA-co-BMA) network was prepared by radical copolymerization of 1.0 mol/l AA with 1.0 mol/l BMA in the presence of 0.01 mol/l AIBN as initiator and 0.02 mol/l MBAA as cross-linker in dimethyl sulfoxide. The reaction mixture was bubbled with nitrogen for 15 min to remove oxygen in the mixture, then injected into the space between two glass plates separated by polyethylene spacers (3 mm thick) or into a cylindrical glass tube of diameter 7 mm. Gelation was carried out at 60 °C for 24 h. After polymerization, the cross-linked P(AA-co-BMA) was immersed in 4,000 ml ethanol–water mixture (50/50 wt%) for 1 week to remove the monomers and uncross-linked polymers, then in a large amount water for 3 weeks, until equilibrium was reached. The sample was divided into two parts. One represented as P(AA-co-BMA) network was still immersed into water. To study the PAA-PVP interaction, the other part was put in the solution of PVP of various initial concentrations (3 ml of the solution per 1 mg of swollen network). The samples were thermostated at 25 °C for 1 week, then immersed into water for 3 h to remove PVP absorbed on the surface of P(AA-co-BMA)/PVP complexes. The relative mass of the sample was characterized by m/m 0 ratio, where m is the mass of P(AA-co-BMA)/PVP complex at the equilibrium state and m 0 is the mass of P(AA-co-BMA) gel equilibrated with water. All specimens were dried under vacuum at room temperature for 7 days.
Composition of complexes
To obtain information on copolymer composition and polymer yield, a sample of the prepared P(AA-co-BMA) gels was quenched and then dried under vacuum at room temperature for 10 days to remove solvent and unreacted monomers. The weight loss, except for the solvent, during the drying process was negligible indicating that the monomer-to-polymer conversion was nearly 100% and that the molar ratio of PAA to PBMA in the copolymer was close to 1:1. The composition of P(AA-co-BMA)/PVP complexes was characterized as follows: by knowing the weight of the dried P(AA-co-BMA) gel before complexation, the weight of the complexes equilibrated with water (the water on the surface of complex disks was adsorbed before weighing) and the weight of the dried complexes, we could calculate the binding degree (μ) of PVP with P(AA-co-BMA) network.
Measurements
FTIR spectra were obtained on a Nicolet 200SXV FTIR spectrometer at a resolution of 2 cm−1. A minimum 16 scans were signal averaged. The dried samples were examined as pressed KBr disks. The thermal analyses were made with a differential scanning calorimeter (Du Pont 9900) over a temperature range from 20 to 180 °C at a heating rate of 10 K/min. The dynamic mechanical analyses were carried out with a Du Pont 983 DMA at a fixed oscillation amplitude of 0.1 mm and under nitrogen gas purging. The frequency of 1 Hz was chosen for all the samples examined. A sheet of sample (0.77 cm wide, 0.22 cm thick, 4.2 cm long) were heated from 30 to 150 °C using a heating rate of 5 K/min. Scanning electron microscopy (SEM; model AMRAY 1000) was used to examine the morphologies of the samples. The compression-moulded sample sheets were fractured in liquid nitrogen, and the resulting fracture surfaces were then coated with gold and carbon.
Results and discussion
Complexes formed between PAA or PMAA and PVP are the most stable of the hydrogen bonded complexes [5]. This stabilization is attributed to the strong intermolecular affinity between the polymer chains, and the hydrogen bonding between the carboxyl groups of PAA or PMAA and carbonyl groups of PVP is mainly responsible for the interpolymer association. Scheme 1 shows the synthesis and the expected structure of P(AA-co-BMA)/PVP complex. The IR spectroscopy indicate hydrogen bonding between P(AA-co-BMA) network and linear PVP. The stretching frequency of C=O for PVP shifts from 1,664 to 1,643 cm−1, and that for PAA shifts from 1,730 to 1,714 cm−1, as shown in Fig. 1.
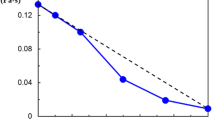
Starodubtzev showed that the absorption of PEG leads to the sharp shrinkage of the gel volume of PMAA or PAA, and the relative mass of the gel is lowered by a factor of 1.5–3.0 [15, 16]. The same phenomenon was reported in [10] for the PVP system. This may be the reason why research work concerning the mechanical properties of complexes has not been presented previously. However, a slight contraction can be observed for the gel of P(AA-co-BMA). Figure 2 (curve 1) illustrates the dependence of the relative mass of the P(AA-co-BMA)/PVP complexes on the initial concentration of PVP (C P). Apparently, this appears to be due to the difference in hydrophobic character of the network. P(AA-co-BMA) is highly hydrophobic compared with PAA or PMAA because it have BMA component in its backbone chain, thus it contain less water in the polymer network. In Fig. 2 (curve 1), we see that the increase of C P leads to a further contraction of the P(AA-co-BMA) gel. It is to be noted that the contraction of the gel in the solution of the linear polymer takes place as a result of the formation of an intermacromolecular gel–polymer complex on the base of hydrogen bonding [15]. Figure 2 (curve 2) illustrates the dependence of the binding degree (μ) on initial concentration of PVP. With increasing C P of PVP solution, the μ value increased simultaneously. This gives the explanation for the conformational changes of P(AA-co-BMA) gel.
DSC and DMA results discussed later suggested that no excess PVP is observed and there is only the PVP/PAA complex phase even in P(AA-co-BMA)/PVP complex formed in the solution of C P = 30 wt%. In Fig. 2, we notice that the P(AA-co-BMA)/PVP complexes have a obviously low μ value compared with the system P(MAA-co-MMA)/PEG [17]. There are two possible reasons for this effect: (1) a first complexation of a carbonyl group of PVP with an acid group of PAA may induce the formation of a second hydrogen bond between vicinal COOH groups, or each carbonyl can be complexed with the two COOH groups simultaneously, as given in ref. [13, 18]; (2) the BMA units in the polymer network are uncomplexable and behave as structure defects.
Differential scanning calorimetry (DSC) is extensively used to investigate miscibility in polymer blends or complexes. A single compositionally dependent glass transition is an indication of full miscibility at a dimensional scale between 20 and 40 nm [19]. Table 1 shows the DSC data of the P(AA-co-BMA)/PVP complexes formed in the solution of PVP of various initial concentrations. All P(AA-co-BMA)/PVP complexes showed only a single glass transition temperature, suggesting that these are fully miscible complexes with a homogeneous amorphous phase. During the heating process, the molecular chain of PAA/PVP complex phase acts as amorphous only during the transfer from the glassy state to the rubbery state.
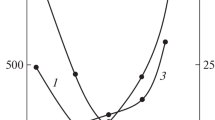
In three-dimensional polymer networks, complexation can significantly affect the network structure [1]. Figure 3 shows the storage modulus of P(AA-co-BMA)/PVP complexes formed in the solution of various PVP concentrations. Modulus ratio data are summarized in Table 1. In Table 1, we see that the dynamic mechanical properties vary apparently between complexed and uncomplexed networks. As previously discussed, the change in the dynamic mechanical properties between the P(AA-co-BMA) network and the P(AA-co-BMA)/PVP complex is primarily due to the cooperative inter-polymer hydrogen bonding which alter the dynamics and structures of the component polymers [17]. Meanwhile, storage modulus ratio of the complexes increased with the PVP concentration, which is in good agreement with the binding degree (μ) results shown in Fig. 2 (curve 2). This is evidence for the conclusion that the mechanic properties of the complexes may be associated with the inter-polymer hydrogen bonding. Loss tangent for the P(AA-co-BMA)/PVP complex is higher than that of P(AA-co-BMA) networks, as shown in Table 1. Since the tanδ corresponds to the strain energy dissipated by viscous friction, a large tanδ implies that the material is more likely to be viscous than elastic. This type of complexes may be used for vibration control due to their high loss tangent value since impact energy can be effectively absorbed [20].
Finally, it would be interesting to obtain a scanning micrograph of the P(AA-co-BMA) before and after complexation with PVP. Figure 4a shows that the P(AA-co-BMA) looks relatively smooth. In Fig. 4b, one can see many holes, which are the result of the absorption of PVP. This might be experimental evidence that the contraction is directly associated with the adsorption of PVP onto the surface of P(AA-co-BMA) networks. The hydrogen bonding between the P(AA-co-BMA) and the PVP may enhance the interfacial adhesion in P(AA-co-BMA)/PVP complexes.
Conclusion
The P(AA-co-BMA)-PVP complexes was studied by DSC, DMA, and SEM. With increasing C P of PVP solution, the binding degree (μ) value increases simultaneously. However, no excess PVP is observed in the P(AA-co-BMA)/PVP complexes even if C P reaches 30 wt%. Storage modulus ratio increases with the PVP concentrations, which is in good agreement with the μ results.
References
Tanaka T (1978) Phys Rev Lett 40:820
Yu Galacev (1999) Using stimuli-responsive polymers in bioseparation. Bioseparation Special Issue 7:175–280
Ito Y, Sugimura N, Oh HK, Imanishi Y (1999) Nat Biotechnol 17:73–75
Lee H, Park TG (1998) Biotechnol Prog 14:508–516
Lowman AM, Peppas NA (2000) Polymer 41:73
Bekturov EA, Frolova VA, Mamytbekov GK (2000) Macromol Chem Phys 201:1031
Yu X, Tanaka A, Tanaka K, Tanaka T (1992) J Am Chem Soc 66:514
Lowman AM, Cowans BA, Peppas NA (2000) J Polym Sci Polym Phys Ed 38:2823
Tsuchida E, Abe K (1982) Adv Polym Sci 45:1
Liu SX, Fang Y, Hu D, Gao G (2001) J Appl Polym Sci 82:620
Bekturov EA, Frolova VA, Bimendina LA (1999) Macromol Chem Phys 200:431
Osada Y (1979) J Polym Sci Polym Chem Ed 17:3485
Iliopoulos I, Audebert R (1988) Eur Polym J 24:171
Osada Y, Sato M (1976) J Polym Sci Part C 14:129
Philippova OE, Karybiants NS, Starodubtzev SG (1994) Macromolecules 27:2398
Starodubtzev SG, Philippova OE (1992) Vysokomol Soedin Ser B 34, N7:72
Cao YP, Guan Y, Peng YX, Chan ASC (2002) J Mater Chem 12:2957
Iliopoulos I, Halary JL, Audebert R (1988) J Polym Sci Polym Chem Ed 26:275
Kuo SW, Chang FC (2001) Macromolecules 34:4089
Lee BS, Chun BC, Chung YC, Sul KI, Cho JW (2001) Macromolecules 34:6431
Acknowledgement
The authors thank the Natural Science Foundation Research Project of Henan Province (China) (Grant No. 2007150014) for financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, G., Guan, C., Zou, W. et al. Swelling behaviors and mechanical properties of polymer gel/poly(N-vinyl-2-pyrrolidone) complexes. J Polym Res 14, 461–465 (2007). https://doi.org/10.1007/s10965-007-9128-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-007-9128-4