Abstract
Free-radical propagation rate coefficients (k p ) at 30°C for the homopolymerization of cyclohexyl methacrylate (CHMA), 4-hydroxybutyl methacrylate (HBMA), 2-hydroxypropyl methacrylate (HPMA), and 2-hydroxyethyl methacrylate (HEMA) were determined to be 1070, 917, 640, and 71.9 (L mol−1s−1), using the rotating-sector method. The k p value increases rapidly, and the value of life time of free radicals (τs) increases smoothly with increasing the alkyl chain length in the hydroxyalkyl pendant group of the monomer. Values for the steady-illumination polymerization rate for CHMA, HBMA and HPMA are much larger than that for HEMA. Comparisons of k p values from different sources were also made.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The determination of propagation rate coefficients (k p ) for certain monomers such as cyclohexyl methacrylate (CHMA), 4-hydroxybutyl methacrylate (HBMA), 2-hydroxypropyl methacrylate (HPMA), 2-hydroxyethyl methacrylate (HEMA) are quite important to both technology and academics. Either the lacking of available data or the existing of inconsistency among various source data leaves ample space for future exploiting.
Scattered propagation rate coefficient data are found for CHMA [1–5] in literature: (k p /k t 1/2) = 0.185 (L/mol-s)1/2 and ΔH = 12.7 kcal/mol in solution polymerization at 44.1°C [2]; k p of 1,190 (L/mol-s) in solution polymerization (using benzene as solvent) at 30°C, determined by using the rotating-sector technique [3]; k p of 510 (L/mol-s) at 60°C from electron spin resonance (ESR) spectroscopic determination [4]; k p of 260 (L/mol-s) obtained from emulsion polymerization at 50°C [5]; and k p of 619, 930, 1,140 and 1,635 (L/mol-s) from the pulse-laser polymerization-size exclusion chromatograph (PLP-SEC) experiment [1] at 30, 40, 50, and 60°C, respectively.
There are propagation rate coefficient data for HEMA: k p of 1,295, 1,986, 2,107, 3,367 and 3,963 (L/mol-s) at 30, 40, 50, 60 and 70°C, respectively, obtained from the PLP-SEC experiment [1]; k p of 200 (L/mol-s) at 60°C, obtained from emulsion polymerization [6]; and (k p /k t 1/2) of 0.35 (L/mol-s)1/2 by a steady photo-polymerization [7].
An analysis performed in an emulsion polymerization of 2-hydroxypropyl methacrylate (HPMA) estimated the propagation rate coefficient to be 4,200 (L/mol-s) at 80°C [8].
Valuable data for CHMA, HEMA, and HPMA have also been obtained by the PLP-SEC) technique [9, 10].
The PLP technique gained its appeal in the determination of k p , due to that it allowed the direct determination of k p from the chain-length distribution of a polymer prepared by periodic laser pulses. However, the weakness of the PLP technique [11] is that it experienced an extremely high variation of radical concentration in the course of one period, while the pseudostationary polymerizations were initiated by periodic laser pulses.
For the PLP data of acrylates published so far, consistent values of k p were obtained only at temperatures below 30°C. This is attributed to the fact that the propagation rate is decreased by the occurrence of mid-chain radicals at higher temperatures, where transfer-to-polymer reaction is followed by slow re-initiation and β-scission [12].
It has been mentioned that the conventional technique of combining (k p 2/k t ) and (k p /k t ) data in the rotating sector technique was inappropriate since k t is chain-length dependent [13–17] and the polydispersity of the terminating chain varied when the respective (k p 2/k t ) and (k p /k t ) data were measured. However, the rotating sector technique was considered competitive to the PLP method, if some care is taken with respect to the choice of experimental conditions. The situation improves in the chain-length dependent termination, such as : (a) for smaller rate of initiation, (b) for higher order points of inflection, (c) if termination is by combination, (d) if the role played by the shorter one of the two chains becomes less dominant [11].
In this study, the rotating sector technique was utilized in determining the k p values for CHMA and the homologues of hydroxyalkyl methacrylates, such as HEMA, HPMA and HBMA. These data would provide crucial clues to reconcile the existing data.
Experimental
Materials and methods
Monomer, 2-hydroxyethyl methacrylate (HEMA, from Across Organics, purity > 96%) was purified by distillation under reduced pressure. Other monomers, such as cyclohexyl methacrylate (CHMA, from Tokyo Chemical Industry Co., Japan, purity = 96%), 2-hydroxypropyl methacrylate (HPMA, from Tokyo Chemical Industry Co., purity = 96.5%), and 4-hydroxybutyl methacrylate (HBMA, from Aldrich Chemical Company, 94% pure) were purified as follows: washed with the same volume of 5% aqueous sodium hydroxide solution, followed by distilled water, dried over anhydrous magnesium sulfate, distilled under reduced pressure in the presence of calcium hydride, and stored at −20°C until used. Both photoinitiator, 2,2′-diethoxy-acetophenoe (DEAP) and inhibitor, 4-methoxyphenol (MEHQ) were purchased from Tokyo Chemical Industry Co., and used as received.
Polymerization
The purified monomer (18 cm3) and initiator (DEAP, 0.10 wt.− %) was mixed in a degas bottle, and after conducting cycles of freezing, degassing and thawing to remove oxygen, the mixture was transferred to dilatometer through a connected tygon tube. The dilatometer was then placed in a constant temperature bath (30 ± 0.01°C). The light source was an ultraviolet (UV) lamp (“Blak-ray,” model B-100AP, with a power of 100 W and a wavelength of 365 nm) and the light was focused into a parallel beam on the dilatometer in the bath. The distance between the light source and dilatometer was 40 cm, unless otherwise specified. The illumination intensity of UV light was determined by using a photometer (i.e., 1330 digital light meter, Tes Electrical Electronic Corporation, Taiwan). A disk was divided into eight equal sectors, and one sector in four was cut out (i.e., the dark-to-light ratio (r) was set to be three for the rotating sector). The disk, interposing between the light source and the dilatometer, was rotated with a small variable-speed motor.
The photopolymerization was conducted under the controlled illumination. The conversion can be obtained from the variation of the level in the dilatometer. At the end of the polymerization, the reaction mixture was short-stopped in a ten-fold volume of methanol in the presence of trace amount of 4-methoxyphenol (MEHQ) inhibitor and then vacuum-distilled at 50°C for at least 24 h until reaching the constant weight to check the final conversion.
Results and discussion
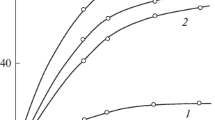
The photopolymerization in bulk has been conducted in this study for CHMA, HEMA, HPMA and HBMA, respectively. The polymerizations were stopped at low conversions to avoid the gel effect. The conversion-time curves for these monomers are shown in Fig. 1. It sounds that the HEMA has the lowest polymerization rate.
Investigation of kp and kt data by the rotating sector method
Analysis of the steady-state kinetic expression allows the determination of the ratio of k p to k t as (k p 2/k t ), where the ratio is given as:
where R p and R i are the rate of propagation and initiation, respectively.
The rotating sector technique makes it possible to decide the average lifetime (τ) of the kinetic chain, where τ is given as
Substituting for [M·]s in terms of the equation of R p = k p [M] [M·]s, the expression for τ becomes
It is possible to calculate the ratio (k p /k t ) by substituting values for ⊺s and R p into Eq. 3. Thereafter, the individual constants of k p and k t can be obtained by comparing the ratio (k p /k t ) with that of (k p 2/k t ).
In each polymerization run, the rotating rate (i.e., deciding the period of illumination) of the rotating sector was varied in a way that the polymerization underwent different steps with different illumination period, as illustrated in Fig. 2. The average rate of polymerization \( {\left( {\overline{{Rp}} } \right)} \) at intermittent illumination with the rotating sector for a particular duration “t” of the light period was compared with that of steady illumination without the rotating sector (Rps). The ratio of \( {\left( {{\overline{{Rp}} } \mathord{\left/ {\vphantom {{\overline{{Rp}} } {Rps}}} \right. \kern-\nulldelimiterspace} {Rps}} \right)} \) (i.e., [M·]/[M·]s) was then plotted against the period of illumination in log–log scale, for an example, HEMA being shown in Fig. 3. The τ value can be obtained by dividing the t value (i.e., the period of illumination) on the experimental fitting line with the t (i.e., t /τ) value on the predicted curve at the same altitude. The τ values obtained for monomers, thereafter, were listed in Table 1.
The initiation rate (R i ) was determined in terms of the ratio of initial inhibitor concentration to induction period. The detailed is referred to [18]. The induction period was measured at each run with different concentrations of inhibitor (MEHQ), where the intercept of the constant-rate line on the abscissa was taken as the induction period, as shown in Fig. 4. Data of the induction time were plotted against those of inhibitor concentration, as shown in Fig. 5. The initiation rate (R i ) was then calculated from the slope of each correlation line in Fig. 5. The R i data are presented in Table 1.
The values of k p and k t can be calculated according to the procedure mentioned earlier, using Eqs. 1 and 3, and the results are shown in Table 1.
To investigate the effect of illumination intensity on the polymerization rate, the UV light source was set at different distances from the reactor to vary the intensity of the light that reaching the reactor. The polymerization rate is plotted against the light intensity in Fig. 6. The order dependence (x) of the polymerization rate (R p ) on the UV light intensity ( [L] ) (i.e., R p ∼ [L]x) for each monomer was close to 0.5. The values are 0.46, 0.56, 0.51 and 0.53, for HEMA, CHMA, HPMA and HBMA, respectively. Note that Fig. 6 offers the relative reactivity among different monomers. This happens to provide valuable base for further comparison.
Hydroxyalkyl pendant group effect
As the homologous hydroxyalkyl methacrylates are concerned, the alkyl chain length in the hydroxyalkyl pendant group of the monomer may have an effect on the data of k p and the life time of free radicals (τs). The k p value increases rapidly, and the τs value increases smoothly with increasing the alkyl chain length in the hydroxyalkyl pendant group of the monomer, as shown in Table 1. In other words, the k p value for each monomer follows a sequence as HEMA < HPMA < HBMA <CHMA . On the other hand, values for the steady-illumination polymerization rate for CHMA, HBMA and HPMA are much larger than that for HEMA, as shown in Figs. 1 and 6.
Values of k p for CHMA [1, 3–5, 9, 19] and HEMA [1, 6, 8, 9] are shown in Table 2 and Fig. 7; and Table 3 and Fig. 8; respectively. The deviations of data between different sources could be quite significant, and could be attributed to factors, such as: difference in conversion where the data were determined, and difference in modes, which deciding the rate-determining steps (e.g., diffusion of radicals and monomers, partition of monomer in different phases, or spurs of radicals generated).
Our HEMA data gaining supports for the smaller kp data of HEMA from Figs. 1 and 6. Obviously, the steady-state polymerization rate data of HEMA are much smaller than other monomers.
Scarce literature data are available for HPMA and HBMA. There is no data for HBMA, but one for HPMA. The data for HPMA are compared in Table 3 and Fig. 9.
Conclusions
Using the rotating-sector method, the free-radical propagation rate coefficients (k p ) at 30°C for CHMA, HBMA, HPMA, and HEMA were determined. The k p value increases rapidly, and the value of life time of free radicals (τs) increases smoothly with increasing the alkyl chain length in the hydroxyalkyl pendant group of the monomer. Values for the steady-illumination polymerization rate for CHMA, HBMA and HPMA are much larger than that for HEMA.
References
Buback M, Kurz CH (1998) Macromol Chem Phys 199(10):2301–2310
Dainton FS, Ivin KJ, Walmlsey DAG (1960) Trans Faraday Soc 56:1784
Yokota K, Kani M, Ishii Y (1968) J Polym Sci, Part A-1 6:1325
Matsumoto A, Mizuta K, Otsu T (1993) J Polym Sci, Part A, Polym Chem 31:2531
Chu H-H, Gau J-H (1995) Macromol Chem Phys 196:2251–2258
Chu H-H, Ou E-D (2000) Polym Bull (Berlin) 44:337
Billaud C, Sarakha M, Bolte M (2000) Eur Polym J 36(7):1401
Chiou H-Y, Thesis MS (1998) Feng Chia University
Hutchinson RA, Beuermann S, Paquet DA, McMinn JH Jr, Jackson C (1998) Macromol 31:1541
van Hert AM (2000) Macromol Theory Simul 9:433–441
Olaj OF, Kornkere A, Zifferer G (1999) Macrmol Theory Simul 8:561–570
van Herk AM (2001) Maromol Rapid Commun 22:687–689
Schulz GV (1956) Z Phys Chem 8:284
Bensen SW, North AM (1959) J Am Chem Soc 81:1339
North AM, Reed GA (1961) Trans Faraday Soc 57:859
Allen PE, Patrick C (1961) Makromol Chem 47:154
Allen PE, Patrick C (1964) Makromol Chem 72:106
Chen HB, Chang TC, Chiu YS, Ho SY (1996) J Polym Sci, Part A: Polym Chem 34:679
Beuermann S, Buback M, Davis TP, García N, Gilbert RG, Hutchinson RA, Kajiwara A, Kamachi M, Lacít I, Russell GT (2003) Macromol Chem Phys 204:1338–1350
Acknowledgment
This study was sponsored by the National Science Council of Taiwan (Grant Number: NSC-92-2216-E-035-008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chu, HH., Wu, JD. Free-radical propagation rate coefficients for hydroxyalkyl methacrylates and cyclohexyl methacrylate. J Polym Res 14, 201–206 (2007). https://doi.org/10.1007/s10965-006-9098-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-006-9098-y