Abstract
Electrolytic conductivity (EC) at low levels (1.0–100 µS·cm−1) is an important measurement parameter of quality for evaluation of the quality of either aqueous or non-aqueous solutions. Certified reference materials (CRMs) are used to calibrate conductivity meters to provide measurements of EC with metrological reliability. Inmetro—the Brazilian National Metrology Institute—has produced CRMs of low EC. An important study is concerned with stability monitoring, since these CRMs generally have short expiration date due to the difficulties usually associated with the stability of low value EC solutions. Inmetro has been performing studies of stability monitoring of low EC CRMs over the last few years by using a primary system of EC. The main goal of this work is to present the results of monitoring the stability of low EC solutions of 1.5, 5, and 50 µS·cm−1 by primary measurements with uncertainties within the characterization uncertainty range of the three CRMs. The results demonstrated that these CRMs remained stable at 25.0 °C during a period of 19 to 20 months.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Electrolytic conductivity (EC) is one of the parameters considered in the international specifications for the quality of water [1, 2] and ethanol fuel [3–5]. EC is a useful analytical tool that can be applied in different fields of science and technology, such as chemicals, pharmaceuticals, power plants, and health care [6]; it is usually measured in aqueous and nonaqueous media using commercial meters.
The measurement of EC is used to monitor the ionic content of solutions (e.g., fruit juices, soft drinks, dialysis fluid, wastewater, fuel) and the purity of water (e.g., natural water, drinking water and water for injection). Many industries rely on certified reference materials (CRMs) of EC to provide measurement results with reliability. The availability of CRMs with accurate and stable EC values is crucial to those industries, as well as for universities and research centers [7]. Low EC CRMs, which are characterized and have their stability monitored by a primary system to guarantee metrological traceability, are needed.
Since 2000, in the first key-comparison of EC coordinated by the Consultative Committee for Amount of Substance (CCQM)-Metrology in Chemistry and Biology, and some national metrology institutes (NMIs) have used the primary method of EC measurement. The theoretical and practical basic knowledge of the measurement of the primary methods for the evaluation of EC in aqueous solutions, carried out in several NMIs, were published [6].
Inmetro, the Brazilian NMI, has been participating in key and pilot comparisons [8–12] of several nominal values of EC since 2007, when the primary system of EC was implemented. Consequently, the Inmetro has obtained calibration and measurement capability (CMC) for different values of EC [13]. All the CRMs of EC produced [14, 15] are characterized by the primary system of EC and, therefore, have contributed to guaranteeing the metrological traceability of EC measurements in Brazil. The CRMs are certified according to ISO Guide 34 [16], and the statistical calculations are performed in accordance with ISO Guide 35 [17].
Currently, the chain of metrological traceability in EC measurements has been established. Two accredited Brazilian laboratories are producing secondary CRMs of EC [18]. These accredited laboratories calibrate their secondary system of EC with the primary CRMs produced at Inmetro. In addition, the laboratories of industries, universities, and research centers can use secondary CRMs to guarantee the reliability and metrological traceability of their measurement results.
However, there is an absence of published results concerning the period of stability for CRMs of low EC. An important study regarding the stability of standard EC solutions was published [7]; nevertheless, the principal goal of the study was to present the results of these solutions for packaging in glass containers (screw-cap bottles, serum bottles, and ampoules). Moreover, in a National Institute of Standards and Technology (NIST) Special Publication [19], a stability study on storing solutions in different containers is presented. In one investigation of low EC standards, the stability study was performed for only 2 months [20]. A recent manuscript described the results of a stability study for a low-value EC CRM during a period of 12 months [21].
In 2001, Linsinger and co-authors [22] postulated that homogeneity and stability studies are crucial characteristics of any CRM, and that the contribution to the uncertainty calculated for these parameters must be conducted according to the Guide to the Expression of Uncertainty in Measurement (GUM) [23]. CRMs for low EC can now be produced successfully to meet the basic criteria of a high-quality technical standard for industrial purposes. The values for these high-quality references have low uncertainties and are traceable to international standards by an unbroken chain of comparison [24].
The primary motivation of this study was that laboratories must prove the reliability and metrological traceability for the results of their EC measurements in different matrices, such as water, ethanol fuel, and other matrices with low EC values. As a result of this monitoring stability study, laboratories will have the expiration date guaranteed for nearly 2 years by primary measurements stated in the CRM certificate.
This monitoring study of the stability of low EC solutions was performed using primary measurements to overcome the limited availability of the literature regarding these standards. This work presents the results of the stability monitoring studies performed by the primary EC system using three CRMs: 1.5 µS·cm−1 (important for low EC samples, such as ultrapure water that is used for pharmaceutical industries), 5 µS·cm−1 and 50 µS·cm−1 (which are very useful for the calibration of conductivity meters and measurement of EC values in beverages and ethanol fuels) at 25.0 °C. The evaluation of the stability monitoring of each CRM was performed based on the measurements obtained during a period of nearly 2 years, and the results were compared with those values obtained in the characterization study and, its respective expanded uncertainty.
This is the first stability monitoring study performed for low EC CRMs over an extended period of time using primary measurements, and this period will impact the expiration date of the CRMs.
2 Materials and Methods
2.1 Equipment
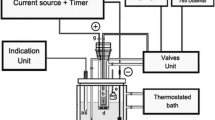
The primary system of EC (Fig. 1) was used for the characterization and stability measurements and is comprised by several calibrated instruments including the primary conductivity cell (Fig. 1a) that contains the sample, a micropositioner system (Heidenhain) (Fig. 1b) responsible for the displacement of one of the Pt electrodes, a super-thermometer (1590 Fluke/Hart Scientific) (Fig. 1c) that monitors the temperature of the sample and a precision inductance (L), capacitance (C) and resistance (R) meter (4284A Agilent) for measurement of resistance of the solution (Fig. 1d). All these devices are parts of the primary system and are interconnected and controlled by a microcomputer (Fig. 1e) [8, 9, 12, 21].
The primary conductivity cell has a cylindrical body made of ceramic material, with two platinum electrodes in each base of the cylinder; one of the Pt electrodes can be electronically moved along the cylindrical body (movable electrode). The calibration of the cell diameter was made according to the following procedure: from the geometric center of the cell, the diameter was measured at four different heights (7, 10, 20 and 30 mm); at each height, 50 evenly spaced points were taken, with five replicates and between two circles around the cell at 1.8° intermediary points were taken, related to the points taken previously. This calibration was made by a measurement system consisting of one coordinate measuring machine (CMM) (Legex 9106) and one laser interferometer system.
The micropositioner system was calibrated by the comparison method, using a calibration standard block (Lamed BP 247) and a CMM (Legex 9106). The procedure is based on the comparison between the values obtained with the displacement meter, and the values measured by the CMM, for ten cycles of measurement in a range from 0 to 24 mm of displacement.
The super-thermometer and a sensor Pt 100 were calibrated by the comparison method using a thermostatic bath with known homogeneity. The temperature of each calibration point was measured with a standard platinum resistance thermometer (SPRT) with a resistance of 100 Ω connected with a standard digital thermometer. The equipment was calibrated in a measurement range from 0 to 30 °C.
A precision LCR meter was calibrated directly by measurement of the standard resistance of an alternating current previously calibrated by the Laboratoire National de Métrologie et d’Essais (LNE), and traceable to the Quantum Hall Effect. The standards resistances used in calibration varied in a range from 10 Ω to 100 kΩ.
For the homogeneity studies of the three CRMs, the EC was measured using a conductivity meter (712, Metrohm) coupled with a conductivity cell (6.0914.040, Metrohm), with a fixed cell constant of 0.105 cm−1, a Pt-100 resistance thermometer (6.1103.000, Metrohm) and a thermostatic bath (E200, Lauda). This equipment was calibrated before the measurements with a CRM with metrological traceability by the primary system of EC.
The mass was determined using an analytical balance (Mettler Toledo, PR 1203, resolution of 0.001 g) and an additional balance (Sartorius, AG, resolution of 0.1 g); all of the values were corrected for air buoyancy. The balances were calibrated using the comparison method between the balance results and several standard weights.
2.2 Materials
The following reagents were used to prepare the CRMs: potassium chloride (KCl), (Merck, lot 8337738504, 99.999 % mass fraction); n-propanol (Tedia, lot 1106508, 99.5 % volume fraction); and hydrochloric acid (HCl) high purity (Merck, lot ZC115818, 30 % mass fraction). All solutions were prepared with deionized water produced by the Purelab Option®(Elga), with an EC value <0.1 µS·cm−1.
For packaging of the CRMs, commercial screw-cap bottles (250 mL) made of borosilicate glass with polypropylene caps, were chosen. All bottles were wrapped with a thin tape of Parafilm® in the cap-bottle junction after the bottles were filled. This type of screw-cap bottle was previously studied [7], and it is recommended for packaging low EC solutions.
2.3 Preparation of the CRMs
Three batches of approximately 40 L of each of the CRMs having nominal EC values of 1.5, 5 and 50 µS·cm−1, respectively, were prepared in glass containers of approximately 46 L. For the CRMs having EC values of 1.5 and 5 µS·cm−1, KCl (0.05893 and 0.22028 g, respectively) was added to a 70:30 v/v mixture of deionized water (28.2 and 28 kg, respectively) and n-propanol (12.1 and 12 kg, respectively). For the CRM having a value of 50 µS·cm−1, a solution was prepared with HCl (0.6409 g) and deionized water (44 kg). Each batch was stirred for 48 h with a magnetic stirrer. Finally, each CRM was packaged in 140 glass bottles (250 mL) and stored in the laboratory at a controlled temperature (21.0 ± 1.0) °C.
2.4 Homogeneity Study
For the homogeneity study, the determination of the EC was applied to 10 bottles that were chosen randomly from the batch, and three replicates of each bottle were made. The one-way analysis of variance (ANOVA) was used to evaluate the within-unit variability and between-unit homogeneity based on ISO Guide 35 [17].
The EC measurements for the homogeneity studies of the three CRMs were done using a conductivity meter, calibrated with CRMs of 5 µS·cm−1 (MRC 8435.0001, Inmetro) and 50 µS·cm−1 (MRC 8846.0002, Inmetro). A 50 mL portion was put into a jacketed glass recipient containing a conductivity cell, a Pt-100 resistance thermometer and a magnetic stirrer. The measurements were taken at a controlled temperature of (25.0 ± 0.2) °C.
It is important to emphasize that the main condition for the homogeneity study is that the method has good repeatability, which is demonstrated by the precision of this method, where the effects related to the heterogeneity can be estimated.
2.5 Procedure
For measuring the EC of each CRM, the solution was introduced into the primary conductivity cell (Fig. 2) and measurements of the resistance for the solution were collected with its respective cell constant value. A sample volume of 160 mL was used for each characterization measurement and also for stability monitoring measurement.
To determine the cell constant used in the measurements, it is necessary take into account the geometrical dimensions of the cell. For the cell used by Inmetro, which has the geometric shape of a cylinder, the constant is given by the relationship shown in Eq. 1, with the distance between the Pt electrodes (or the respective length) divided by the cross-sectional area of the cell.
where Δl is the distance between the Pt electrodes; and D is the diameter of the cross transversal area of the cell.
The distance between the Pt electrodes can be calculated from the variation of the displacement of the Pt electrode from the higher to the lower position (Δl = l H − l L ). The diameter of the cell was previously determined, in the procedure in Sect. 2.1 with a value of 49.9981 mm. The resistance of the solution at (25.000 ± 0.002) °C was calculated by the resistance variation (ΔR = R H − R L) from the higher to the lower position of the Pt electrode.
Each analysis of the EC was averaged for 30 replicates measured for each bottle studied. Equation 2 presents the full formula for the calculation of the EC from the measured resistance taking into account its respective cell constant (which depends on the distance between the Pt electrodes and the diameter of the cell as previously mentioned):
where κ is the EC; l H is the mean of the values of the displacement of the higher position of the movable Pt electrode, l L is the mean of the values of the displacement of the lower position of the movable Pt electrode; R H and R L are the extrapolated resistances at both positions; D is the diameter of the cross transversal area of the cell; α is the temperature coefficient; T m is the mean of the measured temperatures; and T 0 is the reference temperature (25 °C).
For the characterization [16, 17] of the CRMs, three bottles were randomly selected to represent each batch. For the stability monitoring study, one bottle was randomly selected from the batch during the month of the study, and its EC measurement result was compared with the characterization result, considering the expanded uncertainty for each CRM.
2.6 Uncertainty
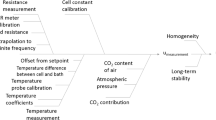
The uncertainty calculation for certification was estimated for each CRM. Equation 3 was used to estimate the combined uncertainty associated with the contributions of the characterization, homogeneity, and stability studies [17]:
where u CRM is the combined standard uncertainty associated with the property value of a CRM; u char is the characterization standard uncertainty; u bb is the between-bottle (homogeneity) standard uncertainty; and u stab is the stability standard uncertainty.
The expanded uncertainty (U) for each CRM was calculated by multiplying the combined standard uncertainty by a coverage factor of k = 2, which corresponds to a confidence level of approximately 95 % [23, 25].
3 Results and Discussion
3.1 Study of the Impedance Spectrum
The frequency range for measuring the resistance of the low EC value solution was established to guarantee its reliable determination [11]. Resistance was measured for each cycle in the frequency range of 20 Hz to 100 kHz and for two positions of the Pt electrode inside the conductivity cell. The value for the best range of measurement was chosen according to the frequency region in which the impedance spectrum is related by the solution resistance.
This condition is important in the cell model used, since the total resistance to the passage of a current in the solution, obtained by electrochemical impedance spectroscopy (EIS) is subdivided into the electrical resistance (the real component of the impedance), the reactance (the imaginary component of the impedance), the geometric capacitance of the cell and the parasitic effects such as polarization, absorption and desorption of ions on the surface of the electrodes.
The resistance of the low EC solutions is based on the mobility of the hydrated ions present in the medium or, similarly, the capability of these ions to carry an electrical current or, inversely, the capability of the solutions to resist the passage of this electrical current. Thus, the resistance must be determined in a way that the components interfering (imaginary component and parasitic effects) are eliminated, or minimized as much as possible.
This is possible because the impedance spectra of the solutions have identified regions where the values of the real component of the measured EIS are stable, regardless of the electrical frequency, within a low and high limit point. In these regions, it was also observed that the imaginary part of the EIS measured has a minimum value. The resistances measured in this region, when extrapolated to the limit of high frequency (or its inverse to zero), can be modelled simply as a parallel circuit between the solution resistance and the electrode capacitance, since the electrical frequency at that point does not interfere with the system. This model leads to the greatest possible minimization of the interfering components in the measure of the real value of the solution resistance and the real component of the impedance.
Figure 3a presents an example of measurements for the CRM having an EC value of 50 µS·cm−1 in which the reactance is plotted versus the resistance in a Nyquist graph [11]. For this low EC value, the frequency of the measurement of the resistance was observed in the range between 200 and 600 Hz. The selected frequencies are illustrated in Fig. 3b in a Bode plot [26].
Similar procedures were applied to the other CRMs studied. The frequency ranges selected for the CRMs of 1.5, 5, and 50 µS·cm−1 were 40–80 Hz, 60–100 Hz, and 200–600 Hz, respectively.
3.2 Certification of the Three CRMs
After choosing the optimum frequency ranges for the EC primary measurements, homogeneity, characterization, and stability studies were performed. The values of the uncertainty for these studies for the certification of the three CRMs are presented in Table 1.
The homogeneity studies of the three CRMs demonstrated that all batches were homogeneous based on the statistical test of ANOVA [17]. The measurement method used (Sect. 2.4) showed good repeatability, while the uncertainty associated with the degree of heterogeneity from the sample (u bb) can be estimated to be equal to the variance observed between the bottles (s bb), which is given by Eq. 4:
where u bb is the between-bottle (homogeneity) standard uncertainty; s bb is the between-bottle variance; MS among is the mean squares difference among the bottles; MS within is the mean squares difference within the bottles; and n 0 is the number of replicates for each unit.
For the characterization of the material performed by a primary method, the property value is usually the average of the measurements done, using three bottles that were chosen randomly from the batch. The uncertainty associated is the result of the combination of uncertainty from the measurement method with the standard deviation of the series of values obtained in these three measurements. This uncertainty is given by Eq. 5:
where s is the standard deviation from the measurements, n is the number of measurements (or bottles), and u meas is the uncertainty associated with the measurement.
One example to estimate the uncertainty of the measurements is presented for the characterization of the CRM having an EC value of 5 µS·cm−1 is presented in Table 2. The descriptions of the main sources of uncertainty incorporated into the measurement method and procedure [8–12, 21] are listed, and these sources were considered in the uncertainty budget. The main sources of uncertainty were the repeatability and the resistance of the measurements. The combined standard uncertainty (u c) of the characterization of the CRM of 5 µS·cm−1 was 5.27 × 10−2 µS·cm−1.
The short-term stability study was performed on previous batches for 1 month, simulating transport conditions at 4.0 and 50.0 °C. These studies demonstrated that the uncertainty associated with short-term stability showed a low contribution to the final uncertainty of the CRM, and was therefore discarded.
On the other hand, the long-term stability was performed over 3 months, according to ISO Guide 34 and ISO Guide 35 [16, 17]. Whether there was a significant trend in the data (EC variations over time), was stidied using a linear regression analysis (linear approximation is a suitable model). When this trend is observed, other analyses are performed. If not, it is expected that this behavior will be repeated throughout the lifetime of the material, and that it will be incorporated in the final uncertainty value of the CRM (Eq. 6):
where u stab is the uncertainty associated to the long-term stability study; s(b 1) is the uncertainty associated with the slope obtained in the linear regression model used; and t is the time studied (usually 3 months).
3.3 Stability Monitoring of the Three CRMs
After certification, the CRMs were monitored over time to confirm their stability. Usually, such measurements are done in secondary and tertiary EC systems, since the primary system is not used in routine tests. Therefore, Inmetro chose to use the primary system of EC measurement for this study, because the low values of the EC need to be investigated under the strictest conditions. Additionally, the expiration date of the CRMs can be extended, when compared with the stability monitoring studies performed using the secondary and tertiary EC systems.
The uncertainty from the characterization (u char) was used as a parameter for the stability monitoring evaluation of each material under study. This procedure is more realistic, in which only the variation of the primary system is considered and not from the CRM uncertainty (u CRM), as shown in Eq. 3.
The primary measurements of the CRMs for the stability monitoring studies were performed for a minimum of 19 and 20 months for the lower EC values of 1.5 and 5 µS·cm−1, respectively. For the higher EC value of 50 µS·cm−1, the period studied was 20 months.
The primary details of the stability monitoring study, including nominal values, type of studies, measurement dates, results, expanded uncertainties (U), and other information are presented in Table 3. Two identifying features are described: characterization and stability monitoring. The characterization is the determination of the property value; the stability monitoring presents the results of the measurements conducted during different periods, subsequent to certification, that correspond to the stability monitoring studies. Usually, one stability monitoring measurement is done quarterly, depending on the value of the CRM. If the material remained stable after 6 months, the measurement of the stability was continued over a shorter period of time to check if any tendency in the stability monitoring was observed.
The individual CRM was considered stable if the result of its measurement was within the value of the expanded uncertainty of the characterization (U), with a coverage factor of k = 2, and a confidence level of approximately 95 %, which was calculated for each CRM. This can be seen graphically in Figs. 4, 5, and 6. Mathematically, the stability monitoring of the three batches was evaluated (Eq. 7) and reported in Table 3. All of the CRMs were considered to be stable during the evaluation period, because the difference between the X CRM and X meas was lower than the square root of the sum of the squares of the combined uncertainty of the characterization, and the combined uncertainty of the value of the CRM measured in the determined month multiplied by k, which is an appropriate coverage factor at a 95 % level of confidence [17]:
where X CRM indicates the certified value (characterization) of the CRM, X meas is the value measured during monitoring, u char is the estimated combined uncertainty of the characterization of the CRM, and u meas is the combined uncertainty associated with the monitoring measurement [17].
The results of the stability monitoring study conducted for the CRM having an EC value of 1.5 µS·cm−1 during a 19 month period after its characterization (Table 3) are illustrated in Fig. 4. The measurements were collected every 3 months for a total of 6 months. Subsequently, the monitoring study was performed over a shorter period of time. Two measurements results were lower when compared with others results, but their values were within the uncertainty of the characterization value of the CRM. The results of this study showed a tendency to approach the upper limit of the expanded uncertainty; however, the CRM remained stable.
The CRM having an EC value of 1.5 µS·cm−1 is extremely low, and the stability results reported in a previous paper [21] were studied only for 12 months. Also, in the same reference, the stability monitoring study was conducted by comparing each value measured with the expanded uncertainty value of the CRM. Therefore, the novelty of this research is that the comparison was based on the more strict expanded uncertainty value of the characterization of the CRM. Nevertheless, the stability monitoring results remained stable under this very strict criterion.
The stability monitoring results for the CRM having an EC value of 5 µS·cm−1 were also stable under the criterion established (Fig. 5). The stability measurements were initiated 3 months after the characterization of the CRM and the CRM continued to be stable after 20 months.
The measurement result with its corresponding expanded uncertainty (U) was (5.13 ± 0.04) µS·cm−1 in the beginning of the study, and was (5.14 ± 0.02) µS·cm−1 after one year. Therefore, even at such a low value, the CRM having an EC value of 5 µS·cm−1 was determined by the primary measurements to be stable during this period.
The value of characterization for the CRM of 50 µS·cm−1 is shown in Table 3. The stability monitoring study results for the CRM of 50 µS·cm−1, performed over a 20-month period, are presented in Fig. 6. The values of the measurements indicate that the CRM was stable during the period studied, because the values are within the expanded uncertainty (U) value of the CRM characterization.
The relative changes in the EC for all of the solutions studied were generally <4.0 % (Fig. 7). This variation over time can be explained by factors such as the leaching of the ions present in the composition of the bottles used for the packaging of the samples; by evaporation of the solvent; and by the solubilization of the CO2 present in the atmospheric air over the time. All of these factors increase the concentration of ionic species present in the medium and, consequently, increase the ionic strength of these solutions. These results have some agreement with those obtained in a previously study [7], which has a large evaluation of conductivity value variation over time for different types of packaging and conductivity values.
However, the same study does not evaluate the issue of the CRM stability in relation to the certification value, which takes into account not only the variation in the absolute values of EC over the time, but also all terms (homogeneity, characterization and short and long-term stability studies) that contribute to the final values for the CRM with its associated uncertainties. This evaluation was performed using Eq. 7 as indicated by ISO Guide 35 [17], where each measurement indicates if the material remains stable in relation to its property value. Although, it should be noted that for these values of EC, lower levels of accuracy are obtained, which does not make its preparation, certification and distribution unfeasible.
This stability monitoring study was conducted by considering the lowest range of expanded uncertainties (U) that were related to the characterization of the three CRMs: ±0.068, ±0.11, and ±0.48 µS·cm−1. The stability monitoring was performed using values within a strict level of uncertainty. Three CRMs evaluated by primary measurements remained stable during a period of 19 to 20 months studied. Thus, the stability monitoring results obtained by the primary system will be relevant for the extension of the expiration time of these CRMs with lower uncertainty.
4 Conclusions
This research evaluated studies of the stability monitoring of CRMs having low EC values performed by the primary system of EC. To the best of our knowledge, no previous paper has presented a stability study for low EC values obtained by primary measurements for a period of time longer than 12 months. The relevance of this work was to demonstrate that low EC CRMs of 1.5, 5, and 50 µS·cm−1 were stable for a period of 19 to 20 months by stability monitoring study. Importantly, the evaluation of stability monitoring was performed by comparing the value of the uncertainty of characterization of each material and not with the expanded uncertainty of the CRMs. Therefore, the proposed research, based on a strict level of uncertainty, was achieved. Conversely, the primary measurements were successfully obtained mainly due to the difficulty of working with solutions of low values of EC. These solutions are important to ensure the highest level of metrological traceability in Brazil and to guarantee the quality of the results of low EC values. Additionally, the results will have applicability in Brazil and to a broader audience.
References
ASTM Standards for Laboratory Reagent Water (ASTM D1193-91). Accessed 17 December 2014
ISO 3696 Standard; CLSI1-CLRW Guidelines; USP: United States Pharmacopoeia. Accessed 10 December 2014
Resolução ANP N° 7, Brasília. http://nxt.anp.gov.br/NXT/gateway.dll/leg/resolucoes_anp/2011/fevereiro/ranp7-2011.xml (2011). Accessed 2 December 2014
Draft prEN 15376 (11/09/2009) (European Community) https://shop.austrian-standards.at/search/Details.action?dokkey=472754&locale=en. Accessed 17 December 2014
ASTM International D4806 (U.S.A.) ASTM D4806—13a Standard Specification for Denatured Fuel Ethanol for Blending with Gasoline for Use as Automotive Spark-Ignition Engine Fuel. http://www.astm.org/Standards/D4806.htm. Accessed 17 December 2014
Brinkmann, F., Dam, N.E., Deák, E., Durbiano, F., Ferrara, E., Füko, J., Jensen, H.D., Máriássy, M., Shreiner, R.H., Spitzer, P., Sudmeier, U., Surdu, M., Vysckocil, L.: Primary methods for the measurement of electrolytic conductivity. Accredit. Qual. Assur. 8, 346–353 (2003)
Shreiner, R.H.: Stability of standard of electrolytic conductivity solutions in glass containers. J. Res. Natl. Inst. Stand. Technol. 107, 393–399 (2002)
Jensen, H.D.: Final Report on CCQM–K36.1 Metrologia 46 Tech. Suppl. 08004 (2009)
Jensen, H.D.: Final Report on CCQM–K36 Metrologia 47 Tech. Suppl. 08025 (2010)
Mariassy, M., Pratt, W.K., Spitzer, P.: Major applications of electrochemical techniques at national metrology institutes. Metrologia 46, 199–213 (2009)
Seitz, S., Manzin, A., Jensen, H.D., Jakobsen, P.T., Spitzer, P.: Traceability of electrolytic conductivity measurements to the international system of units in the sub mS m−1 region and review of models of electrolytic conductivity cells. Electrochim. Acta 55, 6323–6331 (2010)
CCQM-P83 Pilot Study: Measurement of low conductivity (0.5 and 10 mS m−1) in glycerol based solutions. http://www.bipm.org/en/committees/cc/ccqm/pilot_cc.html. (2009) Accessed 2 December 2014
Calibration and measurement capabilities. Amount of substance, pH, Brazil, INMETRO http://kcdb.bipm.org/appendixC/QM/BR/QM_BR_6_7.pdf. Accessed 2 December 2014
Certified reference materials. http://www.inmetro.gov.br/metcientifica/formularios/form_mrc.asp. Accessed 17 December 2014
Fraga, I.C.S., Ribeiro, C.M., Sobral, S.P., Dias, J.C., Gonçalves, M.A., Borges, P.P., Gonzaga, F.B.: Certified reference material of bioethanol for metrological traceability in electrochemical parameters analyses. Talanta 39, 9140 (2012)
ISO Guide 34, General requirements for the competence of reference material producers, Geneva (2009)
ISO Guide 35, Reference materials—general and statistical principles for certification, Geneva (2006)
Accredited producers of reference materials. http://www.inmetro.gov.br/credenciamento/acre_prod_mr.asp. Accessed 17 December 2014
Shreiner, R.H., Pratt, K.W.: Standard reference materials: primary standards and standard reference materials for electrolytic conductivity. http://www.nist.gov/srm/upload/260-142-2ndVersion.pdf (2004) Accessed 18 December 2014
Wu, Y.C., Berezansky, P.A.: Low electrolytic conductivity standards. J. Res. Natl. Inst. Stand. Technol. 100, 521 (1995)
Fraga, I.C.S., Lopes, J.C., Sobral, S.P., Ribeiro, C.M.: Certification of a low value electrolytic conductivity solution using traceable measurements. Accredit. Qual. Assur. 18, 99–104 (2013)
Linsinger, T.P.J., Pauwels, J., van der Veen, A.M.H., Schimmel, H., Lamberty, A.: Homogeneity and stability of reference materials. Accredit. Qual. Assur. 6, 20–25 (2001)
BIPM, IEC, IFCC, ILAC, ISO, IUPAC, IUPAC and OIML (2008) Guide to the Expression of Uncertainty in Measurement–GUM with minor corrections JCGM 100:2008. http://www.bipm.org/utils/common/documents/jcgm/JCGM_100_2008_E.pdf (1995). Accessed 10 December 2014
Arquint, P., Bühler, H., Worbs, S.: Traceable standards for electrolytic conductivity measurements required for pharmaceutical production. CHIMIA Int. J. Chem. 63, 647–649 (2009)
EURACHEM/CITAC Guide quantifying uncertainty in analytical measurement 2nd edn. http://www.eurachem.org/guides/pdf/QUAM2000-1.pdf (2000). Accessed 17 December 2014
Barsoukov, E., MacDonald, J.R.: Impedance Spectroscopy, Theory, Experiment, and Applications. Wiley, New York (2005)
Acknowledgments
The authors are grateful for the financial support of FINEP and CNPq/Prometro.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fraga, I.C.S., Lopes, J.C., Cordeiro, L.R. et al. Evaluation of the Stability of Solutions of Low Electrolytic Conductivity by Primary Measurements. J Solution Chem 44, 1920–1936 (2015). https://doi.org/10.1007/s10953-015-0384-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-015-0384-3