Abstract
The protonation and complex formation equilibria of two biodegradable aminopolycarboxylate chelants {dl-2-(2-carboxymethyl)nitrilotriacetic acid (GLDA) and 3-hydroxy-2,2′-iminodisuccinic acid (HIDS)} with Ni2+, Cu2+, Zn2+, Cd2+ and Pb2+ ions were investigated using the potentiometric method at a constant ionic strength of I = 0.10 mol·dm−3 (KCl) in aqueous solutions at 25 ± 0.1 °C. The stability constants of the proton–chelant and metal–chelant species for each metal ion were determined, and the concentration distributions of various complex species in solution were evaluated for each ion. The stability constants (log10 K ML) of the complexes containing Ni2+, Cu2+, Zn2+, Cd2+ and Pb2+ ions followed the identical order of log10 K CuL > log10 K NiL > log10 K PbL > log10 K ZnL > log10 K CdL for either GLDA (13.03 > 12.74 > 11.60 > 11.52 > 10.31) or HIDS (12.63 > 11.30 > 10.21 > 9.76 > 7.58). In each case, the constants obtained for metal–GLDA complexes were larger than the corresponding constants for metal–HIDS complexes. The conditional stability constants (log10 \( K_{\text{ML}}^{'} \)) of the metal–chelant complexes containing GLDA and HIDS were calculated in terms of pH, and compared with the stability constants for EDTA and other biodegradable chelants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Aminopolycarboxylate chelants (APCs) have been and continue to be used extensively in a variety of industrial processes [1, 2], including the treatment of toxic metal-contaminated solid waste materials [3–5]. APCs are commonly employed to restrict metal ions from playing their normal chemical roles through the formation of stable and water-soluble metal complexes [6, 7]. Because ethylenediaminetetraacetic acid (EDTA) forms stable water-soluble chelant complexes with the majority of toxic metals [2], of the APCs it has been utilized most often. The environmental consequences of the release of APCs to the surroundings has become an issue of concern despite their excellent metal-binding capacities [8]. Remobilization of metal ions from soils and sediments into the aqueous phase may occur when APCs are released into aquatic environments [2]. Lethal exposures resulting from the presence of APCs are likely to persist for a longer period of time because of their poor photo-, chemo- and biodegradability [9–11]. In most cases, an increase in the threshold values of the toxic effects may be observed upon metal complexation [12, 13]. APCs raise the total nitrogen content and phosphate solubility in interstitial waters, and thereby contribute to eutrophication [14, 15].
Legislative regulations have become increasingly stringent about the environmental release of APCs [16, 17], resulting in a wide range of proposals for the treatment of APC-containing wastewater [18, 19]. Alternatively, the search for alternatives to classical APCs in the form of eco-friendly biodegradable variants has become a topic of interest for the treatment of solid waste materials [20–22] or application in the chelant-enhanced phytoextraction of toxic metals [23, 24]. Several biodegradable chelating agents, such as nitrilotriacetic acid (NTA), iminodisuccinic acid (IDSA), [S,S]-ethylenediaminedisuccinic acid (EDDS), methylglycine diacetic acid (MGDA) are considered potential alternatives to EDTA for the aforementioned operations, and the corresponding formation and stability data about their metal–chelant binary complexes are available [25]. The development of the new eco-friendly chelants and the study of their complexation behavior are critical for evaluating the usefulness of these chelants in specific treatment operations [26–29]. dl-2-(2-carboxymethyl)nitrilotriacetic acid (GLDA) and 3-hydroxy-2,2′-iminodisuccinic acid (HIDS) (Fig. 1) are two new commercially available APCs that are supposed to possess eco-friendly characteristics. Furthermore, improved biodegradability of GLDA [30] and HIDS [31], relative to EDTA, has been proposed. The complexation properties of these chelants have not been reported in detail in the standard reference databases of critically selected stability constants of metal complexes. This fundamental information is necessary for assessing new biodegradable chelants for use in a variety of chelant-based industrial cleanup and environmental remediation processes. Therefore, we report on the complexation behavior of GLDA and HIDS with some divalent ecotoxic ions (Ni, Cu, Zn, Cd, and Pb) in aqueous solutions, which will be useful for the design of eco-friendly waste management processes.
2 Experimental Section
2.1 Instrumentation
A KEM AT-610 automatic titrator (Kyoto Electronics, Kyoto, Japan), equipped with a pH-combination electrode and a temperature probe, was used for potentiometric measurements. The electrode system was calibrated with standard buffer solutions (pH 4.0, 7.0 and 9.0) prepared from buffer powders (Horiba, Kyoto, Japan) at 25 ± 0.1 °C, before and after each series of pH measurements. A 100 cm3 titration vessel, equipped with a magnetic stirrer and a water-jacket-type thermostat with a TAITEC EL-8F Coolnit bath water circulator (Saitama, Japan), was used to stir and maintain constant temperature during the titrations. The vessel was sealed with a special cover containing inlets for the electrode, temperature probe, and dosing nozzle for the titrator, in addition to a nitrogen gas inlet and an outlet. Nitrogen gas was used to eliminate the ingress of CO2 and maintain an inert atmosphere.
An iCAP 6300 inductively coupled plasma optical emission spectrometer from Thermo Fisher Scientific (Waltham, MA) was used to determine the metal concentration. The GLDA and HIDS concentrations were validated using an automated TOSOH 8020 high-performance liquid chromatography system from Tosoh (Tokyo, Japan). The Arium® Pro water purification system from Sartorius Stedim Biotech GmbH (Göttingen, Germany) was used to produce the ultrapure water (resistivity >18.2 MΩ·cm).
2.2 Materials
GLDA from AkzoNobel (Amsterdam, Netherlands) and HIDS from Nippon Shukubai (Tokyo, Japan) were used in this study (Fig. 1). Both products were aqueous solutions of their sodium salts, GLDA 40 wt % and HIDS 51.5 wt %. The products are commercially available and were used without any additional treatment.
All of the chemicals and solvents used were of analytical reagent grade. Carbonate-free potassium hydroxide (Kanto Chemical, Tokyo, Japan) was standardized potentiometrically with potassium hydrogen phthalate (Wako Pure Chemical, Osaka, Japan). The solution of hydrochloric acid (Kanto Chemical, Tokyo, Japan) was standardized prior to use. Potassium chloride from Wako Pure Chemical (Osaka, Japan; >0.99 mass fraction purity) was used to adjust the ionic strength of the system. Cadmium(II) chloride, copper(II) chloride dihydrate, and nickel(II) chloride hexahydrate from Kanto Chemical (Tokyo, Japan; >0.99 mass fraction purity), and Titrisol® ampoules of lead and zinc chlorides from Merck KGaA (Darmstadt, Germany), were used to prepare stock solutions of the metals. “CO2-free” water, used to prepare the working solutions, was obtained by boiling and cooling ultrapure water under a stream of nitrogen.
2.3 Software for Computation
The computer program GLEE [32] was used to obtain an estimate of the carbonate concentration of the base by analyzing the results of strong acid–strong base titrations. GLEE was also used to confirm the concentration of the base and the pK w value of water (pK w = 13.78 at 25 ± 0.1 °C, I = 0.1 mol·dm−3). The titration conditions were simulated with the HySS2009 program [33] prior to performing the titrations. The potentiometric data were analyzed using the HYPERQUAD 2008 program [34] to calculate the protonation and metal–chelant stability constants. The HYPERQUAD program facilitates the visual interpretation of refinement, in addition to providing a best fit for the titration data.
2.4 Estimation of Protonation Constants and Metal–Chelant Stability Constants
Aqueous solutions (A–D) of 50 cm3 (total volume) were titrated with 0.1 mol·dm−3 KOH at 25 ± 0.1 °C. The ionic strength of the solutions was maintained constant at 0.1 mol·dm−3 by the addition of an appropriate amount of 1.0 mol·dm−3 KCl stock solution.
-
Solution A: HCl (1.0 × 10−2 mol·dm−3) + GLDA (1.0 × 10−3 mol·dm−3)
-
Solution B: HCl (1.0 × 10−2 mol·dm−3) + GLDA (1.0 × 10−3 mol·dm−3) + M(II) ions (M = Ni, Cu, Zn, Cd, or Pb) (1.0 × 10−3 mol·dm−3)
-
Solution C: HCl (1.0 × 10−2 mol·dm−3) + HIDS (1.0 × 10−3 mol·dm−3)
-
Solution D: HCl (1.0 × 10−2 mol·dm−3) + HIDS (1.0 × 10−3 mol·dm−3) + M(II) ions (M = Ni, Cu, Zn, Cd, or Pb) (1.0 × 10−3 mol·dm−3)
Each solution was allowed to equilibrate for at least 30 min at 25 ± 0.1 °C prior to performing the titration. The auto-titrator recorded the data at constant volume increments and at pre-set intervals, producing real-time titration curves. Each titration was repeated at least three times, and more than 100 points for the potentiometric measurements were utilized in the data analysis.
3 Results and Discussion
3.1 Protonation Constants
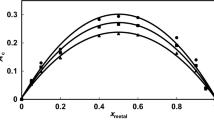
The protonation constants for GLDA and HIDS were calculated from the potentiometric pH profiles of the GLDA- and HIDS-spiked solutions in the absence of metal ions. Raw data for each titration were treated with a non-linear least-squares refinement using the HYPERQUAD program, wherein the weights of the titrant are the independent variables and the pH values are the dependent variables. The percentage distributions of different protonation stages of GLDA and HIDS in the aqueous medium (I = 0.1 mol·dm−3) at 25 ± 0.1 °C are provided in Fig. 2. The proton–chelant constants for the overall reaction, β n , can be described by the following relationship:
where K a1, K a2….K an define the stepwise acid dissociation constants.
The overall (log10 β pqr ) and successive (log10 K) protonation constants for GLDA and HIDS, as calculated by the HYPERQUAD program, are provided in Tables 1 and 5, respectively. The species distribution curves of GLDA and HIDS (Fig. 2) demonstrate that the first protonation of L4− to HL3− occurs at the amino nitrogen atoms in alkaline solutions, and HL3− remains as the dominant species at pH 5.5–8.5 for HIDS (90–99.5 %) and pH 6.0–8.4 (90–98.5 %) for GLDA. The next protonations for GLDA (H2L2− to H4L) and HIDS (H2L2− to H5L+) take place at the oxygen atoms of the carboxylate groups in acidic pH. In GLDA, association of the last proton occurs at pH 2, which is the lower limit of the pH range studied and therefore was not considered in the calculations. The predicted schemes of the protonation equilibria for GLDA and HIDS are provided in Figs. 3 and 4, and are found to be comparable with those reported for other chelants that have analogous structures [35–39]. The formation equilibria and protonation schemes of GLDA and HIDS demonstrate that the respective equilibrium constants depend on either or both of the following factors: (a) the effect of substituent groups, and (b) the space between the functional groups in the chelant structures.
The experimental protonation constant data for GLDA are fairly consistent with data reported for the critically-selected stability constants of metal complexes (shown in parentheses of Table 1) in the NIST database [25], in spite of the variation in the experimental conditions such as ionic strength, background medium and methods of calculation. There are no data for HIDS in the NIST database.
3.2 Metal–Chelant Stability Constants
The overall formation constants (log10 β pqr ) for the binary systems containing metal ions (Ni2+, Cu2+, Zn2+, Cd2+ and Pb2+) and a chelant (GLDA or HIDS), at a molar ratio of metal ion (M) to chelant (L) of one-to-one, were computed from the potentiometric titration data (Tables 2, 3). The hydrolytic behavior (Table 4) of each metal species was taken into account when calculating the metal–chelant stability constants. The overall reactions can be represented by the following general equation:
where p, q and r are the coefficients for metal ions, protons and chelants, respectively, which indicate the stoichiometry associated with the possible equilibria in solution.
The stepwise formation constant (log10 K) for each of the species can be obtained from the differences among the various log10 β values. The log10 K values of GLDA and HIDS are provided in Table 5 and compared with those of NTA, IDSA, EDDS and EDTA. The stepwise formation equilibria are defined by the following equations:
Additional deprotonation reactions involving the coordination of water molecules are defined by the following equation:
The stoichiometries and stability constants of binary metal–chelant complexes were determined from a composition model that was consistent with the titration data, made sense from a chemical point of view, and offered a better statistical fit in comparison with other possible compositions. A good overlap was observed between the experimental and calculated pH values (graphical representations are available as the Supplementary Material), and the refinements of the data sets were obtained throughout the pH range for all the complexes.
Information about the actual metal–chelant species present in aqueous systems under different equilibrium conditions, which are controlled by the pH of the solution, describes the bioavailability of the metals and their corresponding physiological and toxicological behaviors [40]. The formation of protonated MH2GLDA (M = Ni2+, Zn2+, Cd2+ and Pb2+) and MH2HIDS (M = Ni2+, Cu2+, Cd2+ and Pb2+) complexes at various pH values can be observed in the graphical distribution diagrams shown in Figs. 5a (I, III–V) and 5b (I, II, IV, V), respectively. MHGLDA−, MHHIDS−, MGLDA2− and MHIDS2− species are formed under acidic conditions in the presence of Ni2+, Cu2+, Zn2+, Cd2+ and Pb2+. The formation of stable mono-hydroxo complexes, M(OH)GLDA3− and M(OH)HIDS3−, began under neutral conditions, except in the case of Ni2+ with GLDA (Fig. 5a-I). The formation of Cd2HIDS was observed and is attributed to the coordination number of the metal ion being lower than the number of the donor atoms in the HIDS chelant, or alternatively, as a result of steric hindrance [41]. The stability constant data obtained for the complexation between Cu(II) and GLDA are comparable to the values in the NIST database [25]. However, in the NIST database, there are no data for GLDA complexation with Ni2+, Zn2+, Cd2+ and Pb2. Furthermore, the data for HIDS are not included in the same database.
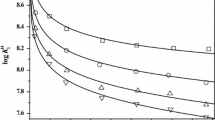
The stabilities of the metal–chelant complexes depend on a number of factors, including the oxidation state and coordination number of the metal ion, as well as the electronic structure and character of the chelant. These factors determine the nature of the bond between metal and chelant, which may be either electrostatic or covalent interactions [42]. The stability of different ML complexes is in the order log10 K CuL > log10 K NiL > log10 K PbL > log10 K ZnL > log10 K CdL in the presence of both GLDA (13.03 > 12.74 > 11.60 > 11.52 > 10.31) and HIDS (12.63 > 11.30 > 10.21 > 9.76 > 7.58). The constants obtained for the metal–GLDA complexes were found to be larger than the corresponding constants for the metal–HIDS complexes.
The stability sequence for the Cu2+, Ni2+ and Zn2+ complexes with GLDA or HIDS follows that of the Irving–Williams series [43]: Ni(II) < Cu(II) > Zn(II). The stabilities of the Pb2+ complex with GLDA or HIDS are higher than those of the corresponding Zn2+ and Cd2+ complexes. A similar trend was also observed for other chelants containing oxygen (of the carboxylic group) as the donor atom, such as TMS (1-hydroxy-3-oxapentane-1,2,4,5-tetracarboxylic acid) and TDS (3,6-dioxaoctane-1,2,4,5,7,8-hexacarboxylic acid) [44].
3.3 Conditional Metal–Chelant Stability Constants
The stepwise or overall formation constant provides fundamental information about the stability of a metal–chelant complex in solution [45]. However, these values do not include factors that are likely to affect the system, such as the pH or the presence of interferences from coexisting species, and are thus rarely applicable for practical purposes [46].
Therefore, the term ‘conditional stability constant’ is defined as the effect of side reactions that may occur during complexation of the chelant with metal ions, such as the effect of chelant protonation and hydrolysis that may occur when a metal ion is in a solution [41]. Various expressions are available for defining the conditional stability constant (log10 K′ML), although the one most frequently used is the following [46]:
where log10 K ML is the formation constant of the 1:1 metal–chelant species. Side reactions involving chelant protonation are expressed by the αHL term. Other interfering reactions, as denoted by the term αM, include the formation of metal hydroxides and the effect of buffer constituents. The formation of metal–chelant–proton species (MLH) or metal–chelant–hydroxide species (MLOH) may also influence the conditional constant for a particular pH and can be taken into account with the term αML in Eq. 7:
The specific form of the equation used for the calculation of conditional constants depends on the incorporation of necessary metal hydroxide species, metal–chelant–proton species, or metal–chelant–hydroxide species in the computation at a fixed pH. Accordingly, Eq. 7 is more frequently used than Eq. 8 [46].
The log10 \( K_{\text{ML}}^{'} \) values of the metal complexes with GLDA, HIDS and other chelants (NTA, IDSA, EDDS and EDTA) were calculated using the binary hydrolysis constants of the metal ions (Ni2+, Cu2+, Zn2+, Cd2+ and Pb2+) (Table 4) and the experimental or literature values of the equilibrium constants. The changes in log10 \( K_{\text{ML}}^{'} \) values with pH are illustrated in Fig. 6. The values of log10 \( K_{\text{ML}}^{'} \) ≥ 6 are considered to be in the suitable complexation range for practical use and, according to this scale, EDTA is appropriate for the target metal ions over a wide pH range of 3–11. GLDA forms stable complexes of practical significance in the pH range 4–11 with Cu2+ and Ni2+, 5–11 with Pb2+, and 6–11 with Cd2+ and Zn2+. For HIDS, the pH range is 4–11 with Cu2+, 5–11 with Ni2+, 6–11 with Zn2+ and Pb2, and 8–11 with Cd2+. We observe that the stabilities of these metal complexes with GLDA or HIDS are lower than those with EDTA, and these complexes also tend to form over a narrower pH range. However, the use of biodegradable APCs is advantageous in terms of environmental safety. The relative stabilities of the metal–chelant complexes of GLDA, HIDS and the other biodegradable APCs (NTA, IDSA, EDDS) at pH 7 is EDDS > GLDA > NTA > HIDS > IDSA for Ni2+, Cu2+, Zn2+ and Pb2+, and GLDA > EDDS > NTA > IDSA > HIDS for Cd2+. The stabilities of metal complexes using HIDS are found to be lower than those using GLDA, which indicates that the GLDA chelant is a better alternative to non-biodegradable APCs in comparison with HIDS. Furthermore, under neutral conditions, the complexation ability of GLDA with divalent metal ions is better than that of NTA and IDSA.
4 Conclusions
The complexion ability of two biodegradable APCs, namely GLDA and HIDS, with ecotoxic metal ions (Ni2+, Cu2+, Zn2+, Cd2+ and Pb2+) in aqueous solutions was investigated experimentally with potentiometric analysis and simulated using the HYPERQUAD computer program. It was found that all of these metal ions formed 1:1 complexes with GLDA and HIDS. The formation of mono- and di-protonated metal complexes occurred under acidic conditions, while mono-hydroxo complexes formed at slightly alkaline pHs. The conditional stability constants for GLDA and HIDS were calculated over the pH range of 2–11, and compared with those of EDTA and other biodegradable chelants (NTA, IDSA and EDDS). The metal–chelant complex stabilities for GLDA and HIDS are lower than those of EDTA, and exhibit a narrower working pH range. However, GLDA and HIDS have advantageous properties due to their lower post-operation ecotoxicity, and are the recommended choices compared with EDTA. The use of GLDA is also advised as the better biodegradable alternative relative to NTA and IDSA in a neutral environment.
References
Conway, M., Holoman, S., Jones, L., Leenhouts, R., Williamson, G.: Selecting and using chelating agents. Chem. Eng. 106, 86–90 (1999)
Nowack, B., VanBriesen, J.M.: Chelating agents in the environment. In: Nowack, B., VanBriesen, J.M. (eds.) Biogeochemistry of Chelating Agents, pp. 1–18. American Chemical Society, Washington, DC (2005)
Raghavan, R., Coles, E., Dietz, D.: Cleaning excavated soil using extraction agents: a state-of-the-art review. J. Hazard. Mater. 26, 81–87 (1991)
Peters, R.W.: Chelant extraction of heavy metals from contaminated soils. J. Hazard. Mater. 66, 151–210 (1999)
Chang, F.-C., Lo, S.-L., Ko, C.-H.: Recovery of copper and chelating agents from sludge extracting solutions. Sep. Purif. Technol. 53, 49–56 (2007)
Leštan, D., Luo, C.L., Li, X.D.: The use of chelating agents in the remediation of metal-contaminated soils: a review. Environ. Pollut. 153, 3–13 (2008)
Hasegawa, H., Rahman, I.M.M., Kinoshita, S., Maki, T., Furusho, Y.: Non-destructive separation of metal ions from wastewater containing excess aminopolycarboxylate chelant in solution with an ion-selective immobilized macrocyclic material. Chemosphere 79, 193–198 (2010)
Rahman, I.M.M., Hossain, M.M., Begum, Z.A., Rahman, M.A., Hasegawa, H.: Eco-environmental consequences associated with chelant-assisted phytoremediation of metal-contaminated soil. In: Golubev, I.A. (ed.) Handbook of Phytoremediation, pp. 709–722. Nova Science Publishers, Inc., New York (2011)
Egli, T.: Biodegradation of metal-complexing aminopolycarboxylic acids. J. Biosci. Bioeng. 92, 89–97 (2001)
Nowack, B.: Environmental chemistry of aminopolycarboxylate chelating agents. Environ. Sci. Technol. 36, 4009–4016 (2002)
Nörtemann, B.: Biodegradation of chelating agents: EDTA, DTPA, PDTA, NTA, and EDDS. In: Nowack, B., VanBriesen, J.M. (eds.) Biogeochemistry of Chelating Agents, pp. 150–170. American Chemical Society, Washington, DC (2005)
Sillanpää, M., Oikari, A.: Assessing the impact of complexation by EDTA and DTPA on heavy metal toxicity using microtox bioassay. Chemosphere 32, 1485–1497 (1996)
Sorvari, J., Sillanpää, M.: Influence of metal complex formation on heavy metal and free EDTA and DTPA acute toxicity determined by Daphnia magna. Chemosphere 33, 1119–1127 (1996)
Horstmann, U., Gelpke, N.: Algal growth stimulation by chelatisation risks associated with complexants in P-free washing agents. Rev. Int. Oceanogr. Med. 260, 101–104 (1991)
Hering, J.G., Morel, F.M.M.: Kinetics of trace metal complexation: role of alkaline-earth metals. Environ. Sci. Technol. 22, 1469–1478 (2002)
van Ginkel, C.G., Geerts, R.: Full-scale biological treatment of industrial effluents containing EDTA. In: Nowack, B., VanBriesen, J.M. (eds.) Biogeochemistry of Chelating Agents, pp. 195–203. American Chemical Society, Washington, DC (2005)
Grundler, O.J., van der Steen, A.T.M., Wilmot, J.: Overview of the European risk assessment on EDTA. In: Nowack, B., VanBriesen, J.M. (eds.) Biogeochemistry of Chelating Agents, pp. 336–347. American Chemical Society, Washington, DC (2005)
Hasegawa, H., Rahman, I.M.M., Nakano, M., Begum, Z.A., Egawa, Y., Maki, T., Furusho, Y., Mizutani, S.: Recovery of toxic metal ions from washing effluent containing excess aminopolycarboxylate chelant in solution. Water Res. 45, 4844–4854 (2011)
Sillanpää, M.E.T., Agustiono Kurniawan, T., Lo, W.-H.: Degradation of chelating agents in aqueous solution using advanced oxidation process (AOP). Chemosphere 83, 1443–1460 (2011)
Tandy, S., Bossart, K., Mueller, R., Ritschel, J., Hauser, L., Schulin, R., Nowack, B.: Extraction of heavy metals from soils using biodegradable chelating agents. Environ. Sci. Technol. 38, 937–944 (2004)
Zhang, L., Zhu, Z., Zhang, R., Zheng, C., Zhang, H., Qiu, Y., Zhao, J.: Extraction of copper from sewage sludge using biodegradable chelant EDDS. J. Environ. Sci. 20, 970–974 (2008)
Tandy, S., Healey, J.R., Nason, M.A., Williamson, J.C., Jones, D.L.: Remediation of metal polluted mine soil with compost: co-composting versus incorporation. Environ. Pollut. 157, 690–697 (2009)
Nowack, B., Schulin, R., Robinson, B.H.: Critical assessment of chelant-enhanced metal phytoextraction. Environ. Sci. Technol. 40, 5225–5232 (2006)
Quartacci, M.F., Irtelli, B., Baker, A.J.M., Navari-Izzo, F.: The use of NTA and EDDS for enhanced phytoextraction of metals from a multiply contaminated soil by Brassica carinata. Chemosphere 68, 1920–1928 (2007)
Martell, A.E., Smith, R.M., Motekaitis, R.J.: NIST Critically Selected Stability Constants of Metal Complexes Database. Texas A&M University, College Station (2004)
Pihko, P.M., Rissa, T.K., Aksela, R.: Enantiospecific synthesis of isomers of AES, a new environmentally friendly chelating agent. Tetrahedron 60, 10949–10954 (2004)
Martins, J.O.G., Barros, M.T., Pinto, R.M., Soares, H.M.V.M.: Cadmium(II), lead(II), and zinc(II) ions coordination of N,N′-(S,S)bis[1-carboxy-2-(imidazol-4yl)ethyl]ethylenediamine: equilibrium and structural studies. J. Chem. Eng. Data 56, 398–405 (2011)
Sari, H., Can, M., Macit, M.: Potentiometric and theoretical studies of stability constants of glyoxime derivatives and their nickel, copper, cobalt and zinc complexes. Acta Chim. Slov. 52, 317–322 (2005)
El-Sherif, A.A., Shoukry, M.M., van Eldik, R.: Complex-formation reactions and stability constants for mixed-ligand complexes of diaqua(2-picolylamine)palladium(II) with some bio-relevant ligands. Dalton Trans., 1425–1432 (2003)
Dissolvine® GL Technichal Brochure. Akzo Nobel Amsterdam, The Netherlands (2004)
Biodegradable Chelating Agent: HIDS. Nippon Shokubai, Osaka (2008)
Gans, P., O’Sullivan, B.: GLEE, a new computer program for glass electrode calibration. Talanta 51, 33–37 (2000)
Alderighi, L., Gans, P., Ienco, A., Peters, D., Sabatini, A., Vacca, A.: Hyperquad simulation and speciation (HySS): a utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 184, 311–318 (1999)
Gans, P., Sabatini, A., Vacca, A.: Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 43, 1739–1753 (1996)
Ichikawa, T., Sawada, K.: Protonation behavior and intramolecular interactions of α, ω-alkanediaminepolymethylenepolyphosphonates. Bull. Chem. Soc. Jpn. 70, 829–835 (1997)
Sanna, D., Bodi, I., Bouhsina, S., Micera, G., Kiss, T.: Oxovanadium(IV) complexes of phosphonic derivatives of iminodiacetic and nitrilotriacetic acids. J. Chem. Soc. Dalton Trans., 3275–3282 (1999)
Sawada, K., Duan, W., Ono, M., Satoh, K.: Stability and structure of nitrilo(acetate-methylphosphonate) complexes of the alkaline-earth and divalent transition metal ions in aqueous solution. J. Chem. Soc. Dalton Trans., 919–924 (2000)
Popov, K., Niskanen, E., Ronkkomaki, H.J., Lajunen, L.: 31P NMR study of organophosphonate protonation equilibrium at high pH. New J. Chem. 23, 1209–1213 (1999)
Buglyó, P., Kiss, T., Dyba, M., Jezowska-Bojczuk, M., Kozlowski, H., Bouhsina, S.: Complexes of aminophosphonates–10. Copper(II) complexes of phosphonic derivatives of iminodiacetate and nitrilotriacetate. Polyhedron 16, 3447–3454 (1997)
Angkawijaya, A.E., Fazary, A.E., Hernowo, E., Taha, M., Ju, Y.-H.: Iron(III), chromium(III), and copper(II) complexes of l-norvaline and ferulic acid. J. Chem. Eng. Data 56, 532–540 (2011)
Ringbom, A.: Complexation in Analytical Chemistry. Interscience Publishers, New York (1963)
Bell, C.F.: Principles and Applications of Metal Chelation. Clarendon Press, Oxford (1977)
Irving, H., Williams, R.J.P.: The stability of transition-metal complexes. J. Chem. Soc., 3192–3210 (1953)
Motekaitis, R.J., Martell, A.E.: Potentiometry of mixtures: metal chelate stability constants of 1-hydroxy-3-oxapentane-1,2,4,5-tetracarboxylic acid and 3,6-dioxaoctane-1,2,4,5,7,8-hexacarboxylic acid. Inorg. Chem. 28, 3499–3503 (1989)
Martell, A.E., Hancock, R.D.: Metal Complexes in Aqueous Solutions. Plenum Press, New York (1996)
Davidge, J., Thomas, C.P., Williams, D.R.: Conditional formation constants or chemical speciation data? Chem. Spec. Bioavail. 13, 129–134 (2001)
Baes, C.F., Messmer, R.E.: The Hydrolysis of Cations. Wiley Interscience, New York (1976)
Acknowledgments
This research was partially supported by the Grants-in-Aid for Scientific Research (K22042) from the Ministry of the Environment, Japan. We thank Professor Peter Gans for his assistance with the HYPERQUAD software. Additionally, the authors (ZAB and IMMR) wish to thank Professor Muhammad Habibullah and Professor Benu Kumar Dey (Department of Chemistry, University of Chittagong, Bangladesh) for their useful comments and suggestions regarding this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Begum, Z.A., Rahman, I.M.M., Tate, Y. et al. Formation and Stability of Binary Complexes of Divalent Ecotoxic Ions (Ni, Cu, Zn, Cd, Pb) with Biodegradable Aminopolycarboxylate Chelants (dl-2-(2-Carboxymethyl)Nitrilotriacetic Acid, GLDA, and 3-Hydroxy-2,2′-Iminodisuccinic Acid, HIDS) in Aqueous Solutions. J Solution Chem 41, 1713–1728 (2012). https://doi.org/10.1007/s10953-012-9901-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-012-9901-9