Abstract
Cobalt (0, 1, 5, and 10 %)-doped ZnS nanoparticles have been synthesized via low-temperature solvothermal technique. The resultant nanoparticles have been analyzed using transmission electron microscope (TEM), electron dispersive spectroscope (EDS), X-ray diffraction (XRD), and UV-visible (UV-Vis.) and photoluminescence (PL) spectrophotometer. Magnetic studies have been carried out using a vibrating sample magnetometer (VSM). The average size of nanoparticles has been found to be ∼9 nm. The XRD spectra indicated cubic phase of the undoped and Co-doped ZnS nanoparticles. The band gap of doped nanoparticles, 4.80 eV, has been found to be blueshifted as compared to its undoped counterpart, 4.59 eV. Room-temperature emission spectra exhibited blue and green lines. Magnetic studies indicated diamagnetic and ferromagnetic character at lower Co concentrations, 0 and 1 %, and ferromagnetic and paramagnetic character at higher Co concentrations, 5 and 10 %.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Semiconducting materials doped appropriately with magnetic materials, such as transition metals or rare-earth metals, are known as dilute magnetic semiconductors (DMS). In the recent times, there has been a tremendous interest in the research on transition metal-doped chalcogenide nanomaterials. The new kind of DMS materials exhibit optical and magnetic behaviors due to charge and spin controllability [1]. Due to its wide band gap, ZnS has been an attractive material, as it acts as a good host for transition metals. The transition metal-doped ZnS nanostructures have a wide variety of applications in the area of spintronics, solar cells, light-emitting diodes, magnetic resonance imaging (MRI), lasers [2–7], etc. Bhargava et al., in 1994, attempted for the first time with Mn-doped ZnS nanocrystals to explore the optical and magnetic properties of ZnS-based DMS nanostructures [8]. Since then, a lot of research has been focused on the structural, optical, and magnetic studies of transition metal-doped ZnS nanostructures. The nanostructures can be specifically used in a variety of applications due to its unique quantum confinement effects. Generally, the properties of materials are size and shape dependent; hence, when doped appropriately with transition metals or rare-earth metals, these ZnS nanostructures can be useful as fundamental building blocks for fabricating the advanced nanoscale electronic and optoelectronic devices. Various studies on photoluminescence and magnetic studies [9–14] of transition metal-doped ZnS nanostructures are available in literature. But very few reports have been found on Co-doped ZnS nanostructures. Lu et al. [15] reported the structural and magnetic behavior of Co-doped ZnS nanowires. It was observed that the Co-doped ZnS nanowires exhibit ferromagnetism at room temperature and ferromagnetism increases as the Co concentration is increased. Sambavisham et al. [16] reported temperature-dependent-induced magnetism in Co-doped ZnS nanoparticles at room temperature. In this study, magnetic hysteresis was observed at room temperature and the magnetization was found to be reduced with increased Co concentration in ZnS host. Patel et al. have reported the grain boundaries induced magnetization at room temperature in Co-doped ZnS thin films [17].
In this report we have tried to analyze the structural, optical and magnetic changes induced in Co-doped ZnS nanoparticles which are indicating mixed magnetic behavior. Till date, no or very few reports have been observed on such mixed magnetic character of Co-doped ZnS nanoparticles.
2 Experimental Details
2.1 Synthesis
All the chemicals were purchased from Sigma-Aldrich. All the reactants and solvents of analytical grade were used without any further purification. Zinc acetate has been used as a source of Zn, thiourea as a source of sulfur, and cobalt chloride as a source of Co, and ethanol has been used as a solvent. For synthesis of ZnS nanoparticles, the solvothermal technique as described in the literature [18] has been used with little modifications. In a typical procedure, zinc acetate and thiourea, in required concentration, were dissolved in the 70 ml ethanol. For cobalt doping, cobalt chloride, in desired concentration, is added to the above mixture. The solution mixture was sealed in a Teflon-lined stainless steel chamber of 100 ml capacity and placed in an oven at a temperature of 180 °C for 10 hours. The resultant mixture was allowed to cool at room temperature and washed with water and acetone for four–five times. The obtained samples were dried for 6 h at 80 °C.
2.2 Characterization
The morphological studies were analyzed with transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) using an FEI Tecnai F30 transmission electron microscope. X-ray diffraction studies were carried out using a PANalytical X’Perto Pro X-ray diffraction machine using copper characteristic wavelength, λ = 1.54 Å. The optical properties were studied by using a fluorescence spectrophotometer (LS55, Perkin Elmer) and a UV-Visible (UV-Vis.) spectrophotometer (Specord-205, Analytik Jena). The elemental analysis was carried out using Oxford analytical system coupled with a scanning electron microscope (SEM). Magnetic studies were carried out by a vibrating sample magnetometer (Lake Shore 7040).
3 Results and Discussion
3.1 Morphological Studies
Morphological analyses were made with the help of TEM and HRTEM. The images of undoped and Co-doped ZnS nanoparticles are shown in Fig. 1.
The interatomic spacing values as observed from HRTEM images have been found to be 3.3 and 3.1 \(\acute {\mathrm {{\AA }}}\) for undoped ZnS and 10 % Co-doped ZnS nanoparticles, respectively. The decreased interatomic spacing values have been confirmed in XRD studies. The presence of doped Co was confirmed by electron dispersive spectroscopic (EDS) analysis. EDS spectra obtained confirm the presence of Co in the host ZnS semiconductor as shown in Fig. 1c, f. The observed doped amount of Co is less as compared to actual doped percentage which may be ascribed to the impurities present in the precursors or washings of samples.
3.2 Structural Studies
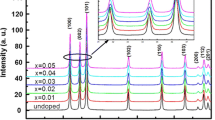
Structural properties have been analyzed using X-ray diffraction (XRD). The patterns obtained are shown in Fig. 2. Peaks have been obtained at 2 θ = (111), (200), (220), and (311) corresponding to the Bragg’s angles of 28.65°, 33.05°, 47.95°, and 57.13°, respectively, accord well with JCPDS data card (JCPDS 80-0020), indicating the cubic phase of the synthesized nanoparticles. Broadening in the XRD peaks indicate the nanometer regime of doped and undoped ZnS nanoparticles.
No extra peak of impurity phase has been observed as no clear peaks of higher intensity have been observed, indicating the single phase of all samples, which confirms the substitution of Co ions in ZnS lattice. The particle size as estimated from Scherrer’s formula [19], given by
where D is the diameter, λ = 1.54 \(\acute {\mathrm {{\AA }}}\) is the wavelength of CuK α radiation, β is the full width at half maxima (FWHM), and θ is the Bragg’s angle. The estimated particle size is ∼9.38 nm which accords well with TEM size. Table 1 shows various lattice parameters of undoped and Co-doped ZnS nanoparticles along the (111) plane.
A decrease in the lattice parameters may be ascribed to strain induced in ZnS lattice with insertion of Co ions in ZnS lattice. The intensity of the peaks is reduced as the doping concentration is increased, which indicates the deterioration of structural quality of ZnS with Co doping.
3.3 Optical Properties
The optical studies have been analyzed using UV-Vis. and photoluminescence (PL) spectrophotometer.
3.3.1 UV-Vis. Studies
UV-Vis. spectra of undoped and Co-doped ZnS nanorods are shown in Fig. 3a. The maximum absorbance in case of undoped ZnS nanorods has been found to be at 270 nm, corresponding to the band gap of 4.59 eV, blueshifted as compared to bulk counterpart.
This blueshift may be ascribed to quantum confinement effects [20]. As the Co concentration in ZnS matrix is increased, the absorbance is found to be blueshift as compared to the undoped ZnS nanoparticles. The maximum absorption (λ max) for 10 % Co-doped ZnS nanorods was found to be 257 nm. This behavior is illustrated in Fig. 3b. The blueshift in Co-doped ZnS nanoparticles may be attributed to fact that the radius of Co ions is smaller as compared to the replaced Zn. This trend may also be ascribed to the formation of new energy levels in the Co-doped ZnS energy band. It again confirms the replacement of Zn ions with doped Co ions in the ZnS lattice.
3.3.2 Photoluminescence Studies
The room-temperature PL emission spectra obtained at the excitation wavelength of 300 nm (λ ex. = 300 nm) are shown in Fig. 4.
The spectra obtained indicate the peak centered at 450 and 525 nm due to blue and green emission, respectively. The broad emission band exhibited at 450 nm may be ascribed to surface defect states originating due to sulfur vacancies [21]. The blue emission may be attributed to radiative recombination between sulfur vacancy-related donor energy levels and purity phase of host ZnS material. Another peak centered at 525 nm may be associated with self-activated zinc vacancies of synthesized ZnS nanoparticles [22]. The photoluminescent intensity increases with the doping of cobalt ions at 1 and 5 % concentrations, but it reduces as the Co concentration is increased to 10 %. The enhancement of photoluminescent intensity may be ascribed to the creation of new radiation centers or size reduction caused by Co doping [23]. As the Co concentration is increased further up to 10 %, the doped Co ions interfere with the radiative recombination, which overshadows the effect of creating new radiation centers, resulting in the quenching of fluorescence intensity at higher doping concentrations. Similar observations have been reported earlier in the literature with Ni doping in ZnS nanorods [9, 24].
3.4 Magnetic Studies
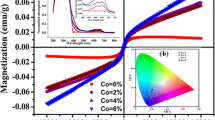
The magnetic behavior of Co-doped ZnS nanoparticles was analyzed using the M-H curves obtained using a vibrating sample magnetometer. The M-H curves obtained are shown in Fig. 5a. The curves clearly indicate the magnetic behavior induced in the Co-doped ZnS nanoparticles.
It is clear that the undoped ZnS nanoparticles exhibit a mixed behavior of diamagnetic and ferromagnetic. As the Co concentration is increased to 1 %, the doped ZnS nanoparticles have been depicting a saturated ferromagnetic character and diamagnetic charter is induced at higher magnetic field. Further increase in Co concentration to 5 and 10 % induces ferromagnetic character at lower magnetic fields and paramagnetic behavior as the field is increased. It is clear that at lower Co concentration, the behavior exhibited is ferromagnetic, but as the concentration is increased further, paramagnetic comes into the picture along with the increased ferromagnetic character but no saturation has been observed till 10 KOe. The coercivity and remanent values are illustrated in Fig. 5b. A decrease in coercivity values indicates the soft magnet character of the doped ZnS nanoparticles, whereas the retentivity is reduced at 1 % Co concentration, but as the concentration increased to 5 and 10 %, a substantial increase has been observed in retentivity of ZnS nanoparticles. This behavior clearly indicates that insertion of Co ions in ZnS matrix induces a mixed ferromagnetic and paramagnetic character in the doped ZnS nanorods. It may be ascribed to the intrinsic coupling [25] between the doped Co atoms and not due to the aggregated Co atoms in the ZnS lattice, as no secondary phases have been observed in the XRD analysis. The results obtained are different than those reported earlier [13], where it has been found that the ferromagnetic character is decreased with increased cobalt ion concentrations due to antiferromagnetic interactions. However, some reports, at higher cobalt ion concentrations, observed ferromagnetic behavior at room temperature [15]. Another possible reason for room-temperature ferromagnetism may be ascribed to the sulfur vacancies and zinc interstitials [26].
4 Conclusions
The undoped and Co-doped ZnS nanocrystals have been synthesized by low-temperature solvothermal technique. The observed doping concentration amount has been found to be less than the actual percentage amount. The synthesized nanoparticles have an average diameter ∼9 nm with cubic phase. A decrease in the lattice parameters may be ascribed to strain induced in ZnS lattice with insertion of Co ions in ZnS lattice. The maximum absorbance in case of undoped ZnS nanorods has been found to be at 270 nm, corresponding to the band gap of 4.59 eV, blueshifted as compared to bulk counterpart. The enhancement of photoluminescent intensity may be ascribed to the creation of new radiation centers or size reduction caused by Co doping in ZnS matrix; further increase in Co concentration decreases the photoluminescent intensity. Magnetic studies indicated mixed diamagnetic and ferromagnetic character paramagnetic at lower Co concentrations, i.e., 0 and 1 %, and mixed ferromagnetic and paramagnetic character at 5 and 10 % Co concentrations in ZnS matrix.
References
Wolf, S.A., Awschalom, D.D., Buhrman, R.A., Daughton, J.M., von Molnar, S., Roukes, M.L., Chtchelkanova, A.Y., Treger, D.M.: Spintronics: a spin-based electronics vision for the future. Science 294, 1488 (2001)
Furdyna, J.K.: Dilute magnetic semiconductors. J. Appl. Phys. 64, R29 (1988)
Steigerwald, M.L., Brus, L.E.: Semiconductor crystallites: a class of large molecules. Acc. Chem. Res. 23, 183–186 (1990)
Kim, D., Min, K.D., Lee, J., Park, J.H., Chun, J.H.: Influences of surface capping on particle size and optical characteristics of ZnS:Cu nanocrystals. Mater. Sci. Eng. B 131, 13 (2006)
Wang, Y., Herron, N.: Nanometer-sized semiconductor clusters: materials synthesis, quantum size effects, and photophysical properties. J. Phys. Chem. 95, 525 (1991)
Colvin, V.L., Schlamp, M.C., Alivisatos, A.P.: Light-emitting-diodes made from cadmium selenide nanocrystals and a semiconducting polymer. Nature 370, 354 (1994)
Bharagava, R.N.: Doped nanocrystalline materials—physics and applications. J. Lumin. 70, 85–94 (1996)
Bhargava, R., Gallagher, D., Welker, T.: Doped nanocrystals of semiconductors—a new class of luminescent materials. J. Lumin. 60, 275–80 (1994)
Jindal, Z., Verma, N.K.: Enhanced luminescence of UV irradiated Zn1−xNixS nanoparticles. Mater. Chem. Phys. 124, 270–273 (2010)
Jindal, Z., Verma, N.K.: Photoluminescent properties of ZnS:Mn nanoparticles with in-built surfactant. J. Mater. Sci. 43, 6539–6545 (2008)
Lotey, G.S., Jindal, Z., Singhi, V., Verma, N.K.: Structural and photoluminescence properties of Eu-doped ZnS nanoparticles. Mater. Sci. Semicond. Process. 16, 2044–2050 (2013)
Li, Y., Cao, C., Chen, Z.: Magnetic and optical properties of Fe-doped ZnS nanoparticles synthesized by microemulsion method. Chem. Phys. Lett. 517, 55–58 (2011)
Kumar, S., Verma, N.K.: Ferromagnetic and weak superparamagnetic like behavior of Ni-doped ZnS nanocrystals synthesized by reflux method. J. Mater. Sci: Mater. Electron. 25, 1132–1137 (2014)
Kumar, S., Chen, C.L., Dong, C.L., Ho, Y.K., Lee, J.F., Chan, T.S., Thangavel, R., Chen, T.K., Mok, B.H., Rao, S.M., Wu, M.K.: Room temperature ferromagnetism in Ni-doped ZnS nanoparticles. J. Alloy Compd. 554, 357–362 (2013)
Lu, M. Y., Chen, L. J., Mai, W., Wang, Z. L.: Tunable electric and magnetic properties of Co x Zn1−x S nanowires. Appl. Phys. Lett. 93, 242503 (2008)
Sambasivam, S., Joseph, D.P., Lin, J.G., Venkateswaran, C.: Doping induced magnetism in Co-ZnS nanoparticles. J. Solid State Chem. 182, 2598–2601 (2009)
Patel, S.P., Pivin, J.C., Chawla, A.K., Chandra, R., Kanjilal, D., Kumar, L.: Room temperature ferromagnetism in Zn1−xCoxS thin films with wurtzite structure. J. Magn. Magn. Mater. 323, 2734–2740 (2011)
Biswas, S., Kar, S.: Fabrication of ZnS nanoparticles and nanorods with cubic and hexagonal crystal structures: a simple solvothermal approach. Nanotechnology 19, 045710 (2008)
Patterson, A.L.: The Scherrer formula for X-ray particle size determination. Phys. Rev. Lett. 56, 978–982 (1939)
Zhang, L., Qin, D., Yang, G., Zhang, Q.: The investigation on synthesis and optical properties of ZnS:Co nanocrystals by using hydrothermal method. Chalcogenide. Lett. 9, 93–98 (2012)
Lee, S., Song, D., Kim, D., Lee, J., Kim, S., Park, I.Y., Choi, Y.D.: Effects of synthesis temperature on particle size/shape and photoluminescence characteristics of ZnS:Cu nanocrystals. Mater. Lett/ 58, 342–346 (2004)
Chen, H., Hu, Y., Zeng, X.: Green photoluminescence mechanism in ZnS nanostructures. J. Mater. Sci. 46, 2715–2719 (2011)
Iqbal, M.J., Iqbal, S.: Synthesis of stable and highly luminescent beryllium and magnesium doped ZnS quantum dots suitable for design of photonic and sensor material. J. Lumin. 134, 739–746 (2013)
Kumar, S., Verma, N.K.: Effect of Ni-doping on optical and magnetic properties of solvothermally synthesized ZnS wurtzite nanorods. J. Mater. Sci: Mater. Electron. 25, 785–790 (2014)
Shafique, M. A., Shah, S. A., Nafees, M., Rasheed, K., Ahmad, R.: Effect of doping concentration on absorbance, structural, and magnetic properties of cobalt-doped ZnO nano-crystallites. Int. Nano Lett. 2, 31 (2012)
Gandhi, V., Ganesan, R., Syedahamed, H.H.A., Thaiyan, M.: Effect of cobalt doping on structural, optical, and magnetic properties of ZnO nanoparticles synthesized by coprecipitation method. J. Phys. Chem. C 118, 9715–9725 (2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, S., Verma, N.K. Room Temperature Magnetism in Cobalt-Doped ZnS Nanoparticles. J Supercond Nov Magn 28, 137–142 (2015). https://doi.org/10.1007/s10948-014-2823-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-014-2823-6