Abstract
Zn1–xCoxO (x = 0.0, 0.06, 0.12, 0.18) nanoparticles were prepared by chemical precipitation method using hydroxyoxalate type precursors and thermal decomposition process. All the samples crystallize in hexagonal wurtzite structure. Cobalt incorporation into the ZnO structure was confirmed by electron diffraction X-ray, transmission electron microscopy and UV–Vis spectroscopy. The increase in the observed band gap of the ZnO nanoparticles compared to that of the bulk ZnO is explained on the basis of size effect. The fluorescence spectra confirm the excitonic peak at 366 nm corresponding to near band edge emission for undoped and cobalt doped ZnO. The ferromagnetism of the lowest concentration cobalt doped ZnO nanoparticle has been evidenced by SQUID. Dielectric properties and magnetoelectric coupling have also been analyzed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The potentiality of exploiting both charge and spin degrees of freedom in the diluted magnetic semiconductors (DMS) have led to the use of these materials in spintronics wherein carrier spins are used to transport store and process information [1–3]. The mutual control of these degrees of freedom is the key in developing devices with new functionalities and better performance. Transition metal (TM) doped wide band gap semiconductors have been found to meet the criterions of DMS. ZnO, an n-type wide band gap semiconductor with a direct band gap of 3.37 eV and having high solubility for doping of TM ions is a potential candidate for the DMS. For real time applications, the requirement is the existence of room temperature ferromagnetism in DMS. The recent predictions [4, 5] that transition metal (Mn, Co, Ni, V, and Fe) doped ZnO exhibit room temperature ferromagnetic behaviors have raised the interest in TM doped ZnO DMS. The magnetic moment per dopant ion and the concentration of the dopant ions is expected to determine the magnetic properties of the DMS. Among the transition metal ions Co2+ ion has almost equal ionic radius as that of Zn2+ and thus has the highest probable solubility in ZnO host crystal. Thus, Zn1–xCoxO is considered as an ideal DMS with huge potentiality for technological applications. Further, ZnO with a large excitonic binding energy of 60 meV has also been used in other electronic and optoelectronic devices. Some of the applications of ZnO pertain to photocatalysis, chemical sensor, varistor, low voltage phosphor material, transparent conductive electrodes in solar cells and flat panel displays etc.
The Curie temperature and the degree of magnetisation i.e. effective magnetic moment per dopant ion depend on the process of fabrication and the particle shape and size. Various chemical and electrochemical synthesis techniques have been used to prepare cobalt doped ZnO nanostructures, such as sol–gel [6, 7], co-precipitation [8, 9], hydrothermal [10, 11], microemulsion [12, 13], pyrosol [14] and electrochemical methods [15]. The chemical precipitation method, as compared with other chemical or physical methods, is an inexpensive method to get a wide range of nanoparticles with controlled sizes and shapes [16, 17]. Compared with the classical method of synthesis based on solid phase reactions, the precipitation method via oxalate intermediates get priority as it allows the control of the particle nucleation and growth from the precursor solution.
Though many papers have appeared since the prediction of room temperature ferromagnetism in TM doped ZnO in the year 2000, we have not come across any report on the mutual coupling magnetoelectric (ME) effect on these compounds. The ME effect is the key in exploiting and mutually controlling the two degrees of freedom in the DMS.
Here we report the optical, dielectric, magnetic and magnetoelectric coupling properties of Zn1–x CoxO nanoparticles prepared by chemical precipitation process, which consists in the simultaneous addition of reactants as acetate salts and oxalic acid, under controlled pH and in the presence of an anti-agglomeration agent. Hydroxyoxalate type precursors are precipitated and transformed into doped ZnO nanoparticles by thermal decomposition process. The calcined precipitates were characterized by XRD, TEM, Fourier transform infra-red (FTIR) spectrum, UV-Vis, FL, dielectric, SQUID and ME coupling measurements.
2 Experimental details
The nanocrystalline Zn1–x CoxO (x = 0, 0.06, 0.12, 0.18) samples were prepared by chemical precipitation method by using Zinc acetate dehydrate and Cobalt acetate tetrahydrate as the starting salts and Oxalic acid as precipitating reagent. Aqueous solutions of acetates (0.5 M) and Oxalic acid (0.5 M) were prepared using deionized water as reaction medium by constant stirring using magnetic stirrer. Following the standard rules, Zinc acetate and different concentrations of Cobalt acetate and Oxalic acid were added by constant stirring. The precipitation was carried out under continuous magnetic stirring while required amount of Ammonium hydroxide solution (NH4OH) was added to maintain the PH at 8 ± 0.2. Tetraethylammoniumhydroxide, (C2H5)4N(OH) was used as an anti-agglomeration agent.
The post-precipitation stages were aged by 1 day and were separated by centrifuge. The centrifuge was set at 6500 rpm for 5 min and the process was repeated 5 times for each sample. The precipitates were dried at 150 °C and finally calcined at 500 °C for 2 h. The calcined compound were ground and compacted into pellets.
3 Characterization techniques
The crystallographic structure of the calcined nanopowders has been analyzed by X-ray diffraction using X-ray diffractometer (RIGAKU, Japan) with CuKα radiation (λ = 1.5418 Å) and scanning angle 2θ between 20° and 80°. The structure and morphology have been analyzed by TEM and SAED. The wurtzite structure is further confirmed by FTIR in open air condition. The optical absorbance spectra have been characterized by Parkin Elmer Lambda-35 UV–Vis spectro-photometer in the wavelength range 300–800 nm. The Fluorescence spectra have been recorded using Perkin Elmer LS 55 Fluorescence Spectrometer with 325 nm Xe Laser excitation of the samples. Dielectric properties have been observed by LCR METRE (HIOKI, Japan). Ferromagnetic behavior of Zn0.94Co0.06O sample has been studied by SQUID at 5 K with the field range ± 4T and the room temperature ME coupling has been taken using a ME setup.
4 Results and discussions
4.1 XRD characterization
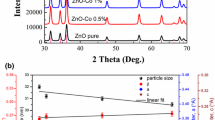
The XRD patterns of the nanopowder samples are presented in Fig. 1. On comparing with the standard JCPDS (JCPDS 89-0510) [18] data, the XRD pattern confirm the hexagonal wurtzite structure with space group P63mc for all the samples. The ZnO structure corresponds to two interpenetrating hcp structures of Zn+2 and O−2 ions displaced along c-axis. The intensity of the diffraction peak corresponding to (101) plane being the highest, suggest preferential growth along [101] direction i.e. c-axis. In case of cobalt doped ZnO additional weak diffraction peaks at 44.83°, 59.24° and 65.06° belonging to Co3O4 secondary phase are detected. The intensity of these peaks increases with increase in cobalt concentration [19]. The average crystallite (coherently diffracted domain) size of the nanoparticles were calculated by Debye–Scherrer equation [20]
where D = crystalline diameter or average grain size of crystallites, k = 0.9 (particle shape factor), λ = wavelength of X-ray radiation and β = FWHM and θ being the Bragg angle.
The calculated average crystallite size corresponding to (100) and (101) peak for different doping concentrations is shown in Table 1. As can be seen from the table, with increase in doping concentration from zero to 18 %, the crystallite size decrease from 25 to 15 nm indicating that the relatively smaller cobalt ion (ionic radius 0.58 Å as compared to Zn ionic radius of 0.60 Å) in the tetrahedral coordination [19] inhibits the growth of crystallites. The systematic decrease in the intensity of the diffraction peaks corresponding to wurtzite structure in the Zn1–x CoxO samples is a consequence of the decrease in the coherently diffracted domain size and is a confirmation on the substitution of Co2+ ions at the Zn2+ ions. The maximum solubility of cobalt in ZnO was reported to be 15 % [21–24]. However, using co-precipitation technique, we could improve the solubility limit of Cobalt in ZnO to 18 %.
4.2 TEM and SAED
The TEM analysis of Zn0.94Co0.06O sample shown in Fig. 2a provides information on the morphological and structural characteristics. The morphology of the sample was found to be hexagonal in nature having average crystallite size 30 nm which matches well with the crystallite size calculated from XRD. The selected area electron diffraction (SAED) pattern is shown in Fig. 2b. SAED pattern exhibit self-organization of nanoparticles and single crystalline behavior of all the nanoparticles in hexagonal pattern. The chemical composition of the material is verified by energy dispersive X-ray (EDX) spectroscopy analysis. Figure 2c shows the EDX spectra of Zn0.94Co0.06O sample which confirms the presence of zinc and cobalt ions in the matrix in agreement with XRD data.
4.3 FTIR spectra
The FTIR spectrum is expected to provide information on the effect of doped cobalt on microstructural and vibrational properties of the hexagonal wurtzite ZnO structure. The FTIR spectra of Zn1–x CoxO samples are shown in Fig. 3a, b. The absorption peak at 424.66 cm−1 is due to stretching mode of Zn–O (metal-oxygen vibration) which confirms the formation of ZnO wurtzite structure in cobalt doped ZnO samples. Two characteristic absorption bands at 602 and 678 cm−1 correspond to Co–O–Co and Co–O–Zn stretching vibration. The intensity of these peaks increases with the cobalt concentration. A small anomaly at 555 cm−1 may be attributed to host lattice distortion such as oxygen vacancies. While the low intensity absorption band at 890 cm−1 correspond to O–C=O bridging oxalate group, the absorption peaks at 1632 and 1389 cm−1 corresponds to the asymmetric and symmetric stretching of the carboxyl group (C=O) [20]. The absorption band at 3434 cm−1 is assigned to the O-H stretching of atmospheric water owing to hygroscopic nature of ZnO. The peaks around 2800 and 2850 cm−1 correspond to the C–H stretching vibrations.
The three major peaks at 424.66, 602 and 678 cm−1 provides structural confirmation and the substitution of the relatively smaller Co2+ ions at the sites of Zn2+ ions in the ZnO host wurtzite structure. The observed stretching vibration of Zn–O at 424.66 cm−1 is in agreement with the accepted wave number of 424 cm−1 in bulk ZnO and indicates that cobalt doping, in the synthesis process we have employed, do not produce strain in the lattice structure.
4.4 UV-visible spectra
The UV-visible absorption spectra in 300–800 nm range of the Zn1–x CoxO series are presented in Fig. 4a. The absorbance spectroscopy is expected to reflect upon the coordination of the dopant ions in the lattice, oxygen vacancies, impurity concentration, optical band gap etc. The spectra exhibit two major peaks around 310 and 396 nm. The one corresponding to the 310 nm correspond to the transition from valence band to conduction band. The optical band gap is calculated from the Tauc plot of \(\left( {\alpha h\nu } \right)^{2}\) Vs \(h\nu\) in Fig. 4b considering the fact that ZnO is a direct band gap semiconductor. The optical band gap of the Zn1–x CoxO samples is found as 3.77, 3.73, 3.69 and 3.57 eV for x = 0.0, 0.06, 0.12 and 0.18 respectively. It is worth pointing that the reported value of optical band gap for bulk ZnO is 3.37 eV. The increase in the observed band gap of the ZnO nanoparticles is assigned to the size effect. It is pertinent to mention here that, the size effect have a bearing on the physical parameters, when the dimensions of the nanoparticles become comparable to the de Broglie wave length of the material. As mentioned earlier, the synthesized ZnO nanoparticle has particle size of 25 nm comparable with the reported de Broglie wave length of 18 nm in the Bulk ZnO [25]. Cobalt doping in the ZnO nanoparticles result in the red shift in band gap energy and is attributed to the sp-d exchange interactions between ZnO band electrons and localized d electrons of the cobalt ions which raises the oxygen 2p valence band and the zinc 4 s conduction band thus decreasing the energy gap [26]. The peak around 396 nm seems to be due to the defect states corresponding to Zn and Co interstitials which lie much below the conduction band. The intensity and width of the peak increase with increase in cobalt concentration. Two mild anomalies at 568 and 659 nm and another substantial peak at 605 nm correspond to the crystal field d-d* transitions of tetrahedrally coordinated Co2+ ions. These peaks confirm the substitution of cobalt ions in tetrahedral sites of wurtzite structure [26]. The small intensity peak at 490 nm has its origin in the oxygen vacancy. Another peak at 690 nm, observed in all the undoped and doped samples, is most likely due to the presence of oxygen interstitials. With increase in the concentration of relatively smaller dopant ions, the number of oxygen interstitials seems to increase [27, 28] resulting in increased intensity of this peak.
4.5 Fluorescence spectra
The Fluorescence spectra of the Zn1–x CoxO series (excited at 325 nm using Xe laser source) at room temperature are shown in Fig. 5. The FL spectra indicate the emission peak at 366, 396, 459, 485 and 530 nm for all cobalt doped ZnO samples. The emission peak at 366 nm (3.39 eV) belongs to the exciton recombination corresponding to near band edge peak of ultraviolet emission [20]. The peak corresponding to 396 nm (3.12 eV) is most likely to be the radiative transition from a neutral donor level of Zn/Co interstitial, which seems to be at 0.27 eV below the conduction band, to the top of the valence band. Two defect level peaks of blue emission at 459 nm (2.7 eV) and at 485 nm (2.56 eV) are detected for cobalt doped ZnO. These blue emission peaks are attributed to the electron transition from donor level of Zn/Co interstitial to acceptor level of neutral Zn/Co vacancy. With increasing cobalt concentration, the peak intensity decreases due to concentration quenching on account of cluster formation [29]. The 530 nm peak is being explained by many authors as the oxygen vacancy donor and the zinc vacancy acceptor recombination [30].
4.6 Dielectric properties
Knowledge of the dielectric behavior of cobalt doped ZnO nanoparticles is necessary for exploiting both the charge and spin degrees of freedom of these nano compounds in devices. For brevity, we present in Fig. 6a the room temperature dielectric dispersion in the range of 100 Hz to 100 kHz. In this range of frequencies all the polarization mechanisms based on electronic, ionic, dipolar and interfacial polarization contribute to the dielectric constant. However, due to inertia, with increase in frequency some of the dipolar modes ceases to follow the field variation and the dielectric constant decreases. From the plot it was observed that the dielectric constant exhibits a peak at around 5 kHz. The graph indicates two different polarization mechanisms being active on either side of 5 kHz frequency. The large value of dielectric constant at lower frequency can be explained on the basis of Maxwell-Wagner mechanism. According to the Maxwell-Wagner mechanism the grain boundary is assumed to be highly insulating compared to the less resistive grain interior. Owing to this mechanism the capacitance of the grain boundary is larger than the capacitance due to the grain interior. The ratio of the relative dielectric constant developed in the nanoparticles due to the grain boundary and the grain interior (bulk) has a ratio of nearly 3 orders (~1000). Further in the synthesized samples of Zn1–x CoxO, the dielectric constant decrease with increase in x.
The dispersion graph of loss tangent in Fig. 6b, indicate low loss in the samples. The loss tangent graph has two contributions.
where the first term explains the mechanism corresponding to the Debye type dipolar relaxation and the second term being the contribution of dc conductivity to the loss tangent. The Debye type relaxation process is characterized by a Gaussian peak in the loss tangent spectrum and the relaxation time (τ), obtained from the condition that at the peak position ωτ = 1, amounts to 2.65 × 10−5 s. The second term due to conductivity is dominant at low frequency and falls sharply with increase in frequency [31, 32].
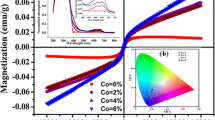
4.7 Magnetic property
Magnetization versus magnetic field (M–H) curve of Zn0.94Co0.06O sample has been measured at 5 K with a maximum applied field of ±4 T by SQUID. Figure 7 exhibit weak ferromagnetic behavior for the sample at very low temperature. The M–H curve can be explained on the basis of the magnetic contribution from the spin orientation owing to exchange interaction of d–d coupling cobalt ions. UV–Vis absorption, FL spectra have indicated that Co2+ ions were successfully incorporated into the wurtzite lattice at the Zn2+ sites. So ferromagnetism is expected to arise from the intrinsic exchange interaction. Sundaresan et al. predicted that the ferromagnetism of the metal oxide nanoparticles, like CeO2, Al2O3, ZnO etc is due to the exchange interaction between localized spin moments generated by the oxygen vacancies at the surface of nanoparticles [33]. Barick et al. exhibited the defect mediated ferromagnetism of self-aggregated ZnO and transition-metal-incorporated ZnO nanostructures [34]. Oxygen vacancies is reported to have a dominant effect in the ferromagnetism of cobalt doped ZnO due to noticeable change in the band structure of the host matrix [35]. The ferromagnetic property of cobalt doped ZnO can also be explained on the basis of the bound magnetic polaron (BMP) model. The electrons locally trapped by oxygen vacancy and the trapped electron occupying an orbital overlapping with the d shells of cobalt neighbors form the BMP [36]. The EDX spectrum shows the oxygen vacancy in the sample. The presences of oxygen vacancies and zinc interstitials have been confirmed in the UV–Vis and FL spectra. With these experimental supports we are inclined to believe that oxygen vacancies and zinc interstitials are the main reasons for ferromagnetism in the cobalt doped ZnO nanoparticles.
4.8 Magnetoelectric coupling
Figure 8 shows the variation of ME voltage co-efficient with DC magnetic field for Zn0.82Co0.18O sample at room temperature. The measured magnetoelectric effect in the materials is essentially due to the coupling between electric polarization and magnetization in the bulk of the sample [37]. In the single phase material, ME coupling arise due to the interaction between the magnetic and ferroelectric sub lattices, but the coupling between them is very weak and occurs at lower temperatures [31]. As has been explained the exchange interaction of d–d coupling with cobalt ions causes magnetic ordering and appearance of room temperature ferromagnetism. Such an interaction produces a shift of the Co2+ ion resulting in improper ferroelectric polarization and the observed ME coupling effect. To get proper ME output, the sample was poled both electrically and magnetically so that the dipole ordering takes place and the sub lattices effectively interact to yield the polarization [38].
ME voltage coefficient, \(\alpha_{E}\), can be determined by using the relation [39, 40],
where, E is the induced electric field, H is the applied magnetic field, V is the ME voltage developed across the sample, ho is the magnitude of the AC magnetic field and d is the thickness of the sample. Here we adopt the dynamic method to measure the ME coefficient in which AC as well as a DC magnetic field was applied simultaneously [41]. The Zn0.82Co0.18O pellet was placed in longitudinal mode and the voltage developed across the sample was measured using a ME set up with a lock-in-amplifier. Experimentally, we applied 15.37 Oe AC magnetic fields and 1 kHz frequency with a DC magnetic field of varying magnitude (−5 to 5 kOe). The AC field was produced by a Helmholtz coil (N = 200 turns with a radius, r = 2.5 cm and coil resistance, R = 23.4 Ω), which was driven by an AC current generated by a function generator. The magnitude of the AC magnetic field (\(h_{o}\)) generated at the centre of the Helmholtz coil can be calculated from the expression:
here, 5 V AC amplitude was applied to the Helmholtz coil resulting in a magnetic field of 15.37 Oe. The ME voltage developed across the sample due to the application of magnetic field can be determined by using the relation [42]:
The total magnetic field can be expressed as: \(H_{total} = H + h_{o} \sin \omega t\). The graphs in Fig. 8 were found to fit nicely with a second order polynomial. From the fitting plot the ME voltage coefficient (α) at zero magnetic field was found to be 8.65 mV/cm. Oe. The second order co-efficient (β) and third order co-efficient (γ) were found to have values 8.79 × 10−6 and −1.56 × 10−9 in appropriate units.
5 Conclusions
The pure and cobalt doped ZnO nanopowders were prepared by the chemical precipitation method at room temperature. The addition of cobalt as a dopant has a large impact on the pure ZnO, with regard to both quality of crystallization and orientation of the crystallites. The average diameter of pure ZnO particles is near about 30 nm. With increase in doping concentration of Co, the particle size decreases. At room temperature, the absorption intensity of the doped samples is higher than the pure one; the optical band gap decreases with increase in Co concentration; the emission peak position of doped ZnO nanoparticles show a small red shift along with enhancement in luminescence performance. These features were attributed to the tune effect of cobalt doping on the energy level of ZnO nanoparticle and increase in the amount of defects because of doping. These properties of cobalt doped ZnO nanoparticles can be used in photonics and opto-electronics devices. The dielectric constant decreases with increasing doping concentration of cobalt. All these materials show low dielectric loss characteristic. SQUID study has showed the ferromagnetic behavior of cobalt doped ZnO nanoparticle. Though several reasons have been attributed for the observed magnetic behavior in the literature, we believe that, in our case the oxygen vacancies and zinc interstitials created by cobalt doping play the key role for the ferromagnetic behavior. Samples with higher concentration of doped materials behave as frustrated magnetic semiconductor. The magnetoelectric coupling coefficient has been found in the order of 8.65 mV/cm.Oe., which is strong enough for exploitation in device applications.
References
C. Ronning, P.X. Gao, Y. Ding, Z.L. Wang, D. Schwen, Appl. Phys. Lett. 5, 84 (2004)
H. Ohno, Science 291(5505), 840–841 (2001)
P. Sharma, A. Gupta, K.V. Rao, F.J. Owens, R. Sharma, R. Ahuja, J.M. Osorio Guillen, B. Johansson, G.A. Gehring, Nat. Mater. 2, 673–677 (2003)
J.J. Beltrán, J.A. Osorio, C.A. Barrero, C.B. Hanna, A. Punnoose, J. Appl. Phys. 113, 17 (2013)
R. Elilarassi, G. Chandrasekaran, Am. J. Mater. Sci. 2(1), 46–50 (2012)
M.K. Lima, D.M. Fernandes, M.F. Silva, M.L. Baesso, A.M. Neto, G.R. de Morais, C.V. Nakamura, A.O. Caleare, A.A.W. Hechenleitner, E.A.G. Pineda, J. Sol-Gel. Sci. Technol. 72, 301–309 (2014)
K. Das, S. Ray, S. Chaudhuri, A.B. Maity, Indian J. Pure Appl. Phys. 47, 377–382 (2009)
N. Hasuike, R. Deguchi, H. Katoh, K. Kisoda, K. Nishio, T. Isshiki, H. Harima, J. Phys.: Condens. Matter 19, 365223 (2007)
C.J. Raj, R.K. Joshi, K.B.R. Varma, Cryst. Res. Technol. 46(11), 1181–1188 (2011)
J. Fidelus, R.R. Piticescu, R.M. Piticescu, W. Lojkowskiand, L. Giurgiu, Z. Naturforsch. 63b, 725–729 (2008)
J. Wojnarowicz, S. Kusnieruk, T. Chudoba, S. Gierlotka, W. Lojkowski, W. Knoff, M.I. Lukasiewicz, B.S. Witkowski, A. Wolska, M.T. Klepka, T. Story, M. Godlewski, Beilstein J. Nanotechnol. 6, 1957–1969 (2015)
J.C. Lin, C.P. Lee, K.C. Ho, J. Mater. Chem. 22, 1270–1273 (2012)
P. Dolcet, M. Casarin, C. Maccato, L. Bovo, G. Ischia, S. Gialanella, F. Mancin, E. Tondello, S. Gross, J. Mater. Chem. 22, 1620–1626 (2012)
O.R. Vasile, E. Andronescu, C. Ghitulica, B.S. Vasile, O. Oprea, E. Vasile, R. Trusca, J. Nanopart. Res. 14(12), 1269 (2012)
L. Ding, R. Zhang, L. Fan, Nanoscale Res. Lett. 8, 78 (2013)
T.M. Hammad, J.K. Salem, R.G. Harrison, Superlattices Microstruct. 47, 335–340 (2010)
S. Suwanboon, P. Amornpitoksuk, A. Sukolrat, Ceram. Int. 37, 1359–1365 (2011)
V. Gandhi, R. Ganesan, H.H.A. Syedahamed, M. Thaiyan, J. Phys. Chem. C 118, 9715–9725 (2014)
A. Mesaros, C.D. Ghitulica, M. Popa, R. Mereu, A. Popa Jr., M. Gabora, A.I. Cadis, B.S. Vasile, Ceram. Int. 40, 2835–2846 (2014)
S. Udayakumar, V. Renuka, K. Kavitha, J. Chem. Pharm. Res. 4(2), 1271–1280 (2012)
Z. Zhang, Q. Chen, H.D. Lee, Y.Y. Xue, Y.Y. Sun, H. Chen, F. Chen, W.K. Chu, J. Appl. Phys. 100, 043909 (2006)
C. Rath, P. Mallick, D. Pandey, D. Sa, A. Banerjee, N.C. Mishra, J. Phys.: Condens. Matter 21, 075801 (2009)
S.V. Bhat, F.L. Deepak, Solid State Commun. 135, 345–347 (2005)
S. Lee, B.S. Kim, S.W. Seo, Y.C. Cho, S.K. Kim, J.P. Kim, I.K. Jeong, C.R. Cho, C.U. Jung, H. Koinuma, S.Y. Jeong, J. Appl. Phys. 111, 07C304 (2012)
A.A. Mosquera, D. Horwat, A. Rashkovskiy, A. Kovalev, P. Miska, D. Wainstein, J.M. Albella, J.L. Endrino, Nat. Sci. Rep. 3, 1714 (2015)
R. He, B. Tang, C. Ton-That, M. Phillips, T. Tsuzuki, J. Nanopart. Res. 15, 2030 (2013)
H. Kaftelen, K. Ocakoglu, R. Thomann, S. Tu, S. Weber, E. Erdem, Phys. Rev. B 86, 014113 (2012)
N.H. Alvi, K. ul Hasan, O. Nur, M. Willander, Nanoscale Res. Lett. 6, 130 (2011)
A. Sivagamasundari, R. Pugaze, S. Chandrasekar, S. Rajagopan, R. Kannan, Appl. Nanosci. 3, 383–388 (2013)
F. Wen, W. Li, J.H. Moon, J.H. Kim, Solid State Commun. 135, 34–37 (2005)
S. Sahoo, P.K. Mahapatra, R.N.P. Choudhary, J. Phys. D Appl. Phys. 49, 035302 (2016)
S.A. Ansari, A. Nisar, B. Fatma, W. Khan, A.H. Naqvi, Mater. Sci. Eng., B 177, 428–435 (2012)
A. Sundaresan, R. Bhargavi, N. Rangarajan, U. Siddesh, C.N.R. Rao, Phys. Rev. B 74, 161306(R) (2006)
K.C. Barick, M. Aslam, V.P. David, D. Bahadur, J. Phys. Chem. C 112, 15163–15170 (2008)
S. Kumar, Y.J. Kim, B.H. Koo, H. Choi, C.G. Lee, J. Korean Phys. Soc. 55(3), 1060–1064 (2009)
B. Pal, P.K. Giri, J. Appl. Phys. 108, 084322 (2010)
Y. Zhang, J.P. Zhou, Q. Liu, C.Y. Deng, Ceram. Int. 40, 5853–5860 (2014)
M.M. Kumar, A. Srinivas, S.V. Suryanarayana, G.S. Kumar, T. Bhimasankaram, Mater. Sci. 21(3), 251–255 (1998)
M. Shi, R. Zuo, Y. Xu, L. Wang, C. Gu, H. Su, J. Zhong, G. Yu, Ceram. Int. 40, 9249–9256 (2014)
D. Dipti, S. Juneja, S. Singh, K.K. Raina, C. Prakash, Ceram. Int. 41, 6108 (2015)
P. Pahuja, C. Prakash, R.P. Tandon, Ceram. Int. 40, 5731–5743 (2014)
G.V. Duong, R. Groessinger, M. Schoenhart, D. Bueno-Basques, J. Magn. Magn. Mater. 316, 390–393 (2007)
Acknowledgments
Authors are thankful to the CRF, IIT Kharagpur for TEM and SQUID study. This work is partly supported by minor research project (Grant No. PSW 129/13-14) from UGC of India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samanta, A., Goswami, M.N. & Mahapatra, P.K. Structural, optical, dielectric, magnetic and magnetoelectric properties of Co-doped ZnO nanoparticles. J Mater Sci: Mater Electron 27, 12271–12278 (2016). https://doi.org/10.1007/s10854-016-5385-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5385-5