Abstract
Cobalt ferrite nanoparticles were coprecipitated in air medium using NH3, and the effects of temperature and base concentration on the properties were studied. X-ray diffraction (XRD) technique and Fourier transform infrared spectroscopy were used to investigate the structural properties of the samples. Particle sizes and shapes were determined by a transmission electron microscope (TEM). Magnetic measurements were done using vibrating sample magnetometer at room temperature. The proper reaction temperature was found to be 80 °C for the synthesis of superparamagnetic cobalt ferrite nanoparticles. The effect of base concentration on the properties of the superparamagnetic nanoparticles was investigated under this temperature. The magnetization values of cobalt ferrite nanoparticles increased as the base concentration increased and reached to a value of 32.4 emu/g with zero coercivity. The particle sizes (d XRD, d TEM, and d VSM) of cobalt ferrite nanoparticles were calculated from XRD patterns, TEM images, and magnetic data, respectively. It was observed that the d TEM and d VSM are similar to each other, and the d XRD are bigger than those with the similar trend of increase with the increase of base concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Magnetic nanoparticles have attracted much interest due to their useful properties in many technological areas, such as magnetic recording [1], magnetic fluids [2], MRI contrast agents [3], hyperthermia treatment [4], and targeted drug delivery [5, 6]. Ferrite nanoparticles (Fe3O4, CoFe2O4, NiFe2O4, ZnFe2O4, etc.) have different applications because of their different properties. Among these ferrites, cobalt ferrite nanoparticles have useful properties such as high magnetocrystalline anisotropy and large magnetostrictive coefficients, for magnetic recording and hyperthermia applications [7]. Besides, cobalt ferrite nanoparticles can show superparamagnetism when the particle size is below a critical value of ∼6–8 nm as reported in [7]. Coprecipitation, hydrothermal synthesis, and thermal decomposition are the main synthesis techniques to obtain cobalt ferrite nanoparticles. Among them, coprecipitation is a simple and economical way to obtain superparamagnetic nanoparticles [8, 9]. In this technique, cobalt ferrite nanoparticles are synthesized from a solution containing Co+2 and Fe+3 ions. The particle sizes and magnetic properties of the nanoparticles can be tailored by changing reaction conditions, such as reaction temperature, base concentration, and annealing temperature. There has been limited number of studies on reaction temperature and base concentration.
Kim et al. [10] coprecipitated cobalt ferrite nanoparticles at different temperatures by using NaOH and investigated the structural and magnetic properties. Zhang et al. [11] synthesized cobalt ferrite nanoparticles by changing NaOH concentration. In [12], different concentrations of NaOH were used for the coprecipitation at room temperature.
In this study, the cobalt ferrite nanoparticles were synthesized by coprecipitation after obtaining the proper reaction temperature. And, the properties of the nanoparticles were investigated. It is seen that the synthesized cobalt ferrite nanoparticles are superparamagnetic and a quite high magnetization value of 32.4 emu/g was obtained.
2 Experimental
For the co-precipitation, 2.38 g cobalt chloride hexahydrate (CoCl2.6H2O, Fluka > 98 %) and 5.48 g ferric chloride hexahydrate (FeCl3.6H2O Sigma Aldrich > 99 %) were used. Ammonium hydroxide (NH4OH, Merck 25 %) was used for the base source. One hundred milliliters NH3 solution was added to 100 ml ion salt solution under vigorous stirring. The reaction was performed in air medium for 60 min. After the reaction, the precipitate was washed three times with distilled water and was dried in an oven. The reaction temperature was changed from 20 to 80 °C. And then the base concentration was changed between 0.5 and 9.0 M at a fixed temperature. The crystal structure of the nanoparticles was investigated with X-ray diffractometer (XRD, PANanalyticals X’Pert PRO) between 20° and 80° by using Cu Kα radiation (λ = 1.54056 Å). Fourier transform infrared spectroscopy (FT-IR, PerkinElmer) was also used for the structural analysis and the spectrum was recorded at 400–1400 cm−1 range using KBr pellets. Particle sizes and shapes were investigated using high-resolution transmission electron microscope (HRTEM, FEI TECNAI G2 F30 model). The physical particle sizes, d TEM, were manually counted and calculated from the TEM images by using Image J programme after taking the TEM pictures of nanoparticles. Magnetic measurements were done by a vibrating sample magnetometer (VSM, ADE Technologies EV9) at room temperature between −20 and + 20 kOe with 1 Oe intervals. The magnetization value of the sample at the highest applied field was denoted as M max in whole text.
3 Results and Discussion
It is known that the reaction temperature is a fundamental parameter to synthesize cobalt ferrite (CoFe2O4) nanoparticles [10]. Under study, obtaining superparamagnetic CoFe2O4nanoparticles is an important point for the potential applications that require a magnetic switching property [13]. Therefore, using 1.5 M NH3, the synthesis was performed at different temperatures (20, 40, 60, and 80 °C) to decide the appropriate reaction temperature. Black precipitate of CoFe2O4 was obtained at 80 °C. Magnetic measurements of the samples were performed by vibrating sample magnetometer (VSM) and the magnetization curves are presented in Fig. 1. As seen from the figure, the samples synthesized at 20, 40, and 60 °C show paramagnetic behavior and the sample at 80 °C show superparamagnetic character. The M max values of the samples synthesized at 20, 40, and 60 °C are 2.4, 3.8, and 5.4 emu/g, respectively. The nanoparticles synthesized at 80 °C have higher M max of 19.9 emu/g, and their coercivity, H c, is zero. In the study [10], CoFe2O4 nanoparticles synthesized at 20 °C showed paramagnetic structure with 2.0 emu/g magnetization value at 10 kOe, which is similar to our work. In the same study, the nanoparticles are superparamagnetic with 4.2 emu/g magnetization value at 40 °C and ferrimagnetic (H c values are 39 and 193 Oe at 60 and 80 °C, respectively) above 40 °C. And, the authors say that there is a considerable change in crystallinity and average particle size between the reaction temperatures 40 and 60 °C. However, in our study, clear X-ray diffraction (XRD) patterns of the nanoparticles can only be obtained at 80 °C (see below discussion). And, superparamagnetic CoFe2O4 nanoparticles can only be obtained at 80 °C. Thus, the reaction temperature was selected as 80 °C and the effect of base concentration was studied accordingly.
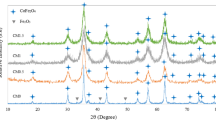
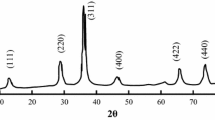
In order to investigate the effect of base concentration on the properties of CoFe2O4 nanoparticles, base concentration was changed from 0.5 to 9.0 M. The initial base concentrations and the measurements of CoFe2O4 nanoparticles are presented in Table 1. The XRD patterns of the samples S1, S3, S7, and S8 were selected and given in Fig. 2. It is observed in Fig. 2a that sample S1 shows amorphous-like structure with no XRD peaks which may be caused by the small particles size of the nanoparticles. In the patterns of S3, S7, and S8, however, the (311), (400), (511), (440), and (533) peaks were observed at 2θ ≈ 35, 44, 57, 62, and 74° , respectively. The observed peaks are the characteristic peaks of CoFe2O4 according to the JCPDS 22-1086 card. The mean crystal sizes, d XRD, were calculated from (311) peak in the patterns using Scherrer’s equation [14], and the results were summarized in Table 1. The d XRD were 7.3, 7.1, 9.9, and 14.9 nm for S3, S6, S7, and S8, respectively. The particle sizes obtained from XRD patterns increases with increasing base concentration.
For further information about the structure of the samples, Fourier transform infrared spectroscopy (FT-IR) analysis was done and the spectra of some samples are shown in the 1400–400 cm−1 region in Fig. 3. For all samples, the transmittance peak is observed at about 590 cm−1. The peak in the spectrum of S1 is very weak, and its intensity is higher for the other samples. The peaks in the range of 500–600 cm−1 indicate the intrinsic stretching vibration of M–O bond, where M corresponds to the metal ion at the tetrahedral site [15]. Thus the peak observed in the spectrum implies the formation of CoFe2O4 [15–17]. The final pH of the solutions after coprecipitation is between 8.6 and 11.1. These pH values are suitable for the precipitation process since a complete precipitation takes place at the pHs between 8 and 14 as indicated in [18]. It can be deterred from the structural analysis that base concentrations from 1.0 to 9.0 M are suitable to obtain CoFe2O4 nanoparticles.
TEM images were obtained to investigate the particle size and shape of the samples. TEM images are given in Fig 4. The physical particle sizes, d TEM, of CoFe2O4 nanoparticles were also determined by TEM analysis and given in Table 1. The d TEM are 2.4 ± 1.0, 5.3 ± 1.2, 6.3 ± 1.5, 5.8 ± 1.8, and 7.5 ± 1.5 nm for samples S1, S3, S6, S7, and S8, respectively. The d TEM increases as the base concentration increases from 0.5 to 6.0 M, and then it decreases at 8.0 M and increases again at 9.0 M. By looking at the XRD pattern, FTIR spectrum, and TEM image of S1, it can be said that at the lowest base concentration particle formation is on the first stage and the growth process is not completed. The calculated d XRD values are bigger than d TEM values; however, the similar trend of increase with increasing base concentration is observed.
Magnetization curves of the nanoparticles synthesized with various base concentrations are presented in Fig. 5. All samples except for S8 are superparamagnetic with zero coercivity. A large amount of increase in the M max from 7.2 to 16.3 emu/g is observed as the base concentration increases from 0.5 to 1.0 M. And, the M max continues to show smaller steps of increase from 16.3 to 32.4 emu/g with increasing base concentration from 1.0 to 6.0 M. This is followed by a little decrease to 28.7 emu/g when the sample was synthesized at 8.0 M, and a slight increase to 31.9 emu/g with a little H c value (4 Oe) is observed at 9.0 M. In [8], superparamagnetic CoFe2O4 nanoparticles have magnetization of 4.2 emu/g at 10 kOe applied field and the other samples with higher magnetization values (36 and 58.3 emu/g) show H c (39 and 193 Oe). Jovanovic et al. [19] synthesized CoFe2O4 nanoparticles by coprecipitation, and they found the magnetization value as 3.2 emu/g and H c as 3.9 Oe. In another study [11], all samples show H c values as high as 5628 Oe and the sample with maximum magnetization (36.24 emu/g) has H c of 673 Oe. Also in [16], as-synthesized CoFe2O4 nanoparticles show high values of H c (2100 Oe) and saturation magnetization (43 emu/g). Oleic acid-coated CoFe2O4 nanoparticles were studied in [20], and they showed H c values higher than 200 Oe. Under study, the main point is to obtain superparamagnetic CoFe2O4 nanoparticles with high M max values. The obtained M max values are close to that of CoFe2O4 nanoparticles with ferrimagnetic character [11–20]. More importantly, the M max values of our nanoparticles are quite higher than the values obtained for superparamagnetic CoFe2O4 nanoparticles [10].
The mean magnetic sizes, d MAG, were calculated according to the equation in [21] and listed in Table 1. Magnetic size increases from 2.9 ± 0.3 to 4.2 ± 0.2 nm as the base concentration increases from 0.5 to 1.0 M and then slightly increases and stays almost constant as the base concentration keeps increasing, see Table 1. The d VSM and d TEM values are close to each other, and d XRD values are larger than those. It might be inappropriate to directly correlate the d XRD values and properties of the samples since the XRD patterns showed high noises. However, the similar trend of change is observed on the d XRD, d TEM, and d VSM and hence the corresponding properties. Besides, the d TEM was taken into consideration to further explain the properties of the nanoparticles. The d TEM of superparamagnetic CoFe2O4 nanoparticles are between 2.4 ± 1.0 and 6.3 ± 1.5 nm, and the size of the nanoparticles showing coercivity is 7.5 ± 1.5 nm. These values are in good agreement with the study [7], saying that the superparamagnetic particle size limit of CoFe2O4 nanoparticles is ∼ 6–8 nm.
A study using different NaOH concentrations showed that the size of CoFe2O4 nanoparticles with coercivities increases as the base concentration increases; however, the saturation magnetization slightly changes [22]. In our study, the M max of the superparamagnetic CoFe2O4 nanoparticles increases from 7.2 to 32.4 emu/g with increasing the d TEM from 2.4 ± 1.0 and 6.3 ± 1.5 nm. Due to the increase of surface to volume atomic ratio for small particles, the surface canting of magnetic moments increases and magnetization value decreases as stated in [23, 24]. Therefore, the M max is lower for the small nanoparticles and increases as the particle size increases from 2.4 ± 1.0 to 6.3 ± 1.5 nm with the increase of base concentration. For the sample S7, the M max slightly decreases with a correlation of particle size.
4 Conclusions
Cobalt ferrite nanoparticles were synthesized in air medium using NH3 as base source. Their characterizations were made according to the reaction temperature and base concentration. In order to comprehensively investigate the structure of the synthesized samples, X-ray diffraction and Fourier transform infrared spectroscopy were performed. It was observed that the superparamagnetic cobalt ferrite nanoparticles can only be synthesized at 80 °C. At this temperature, the effect of base concentration on the properties of the nanoparticles was investigated. It is seen that the particle sizes and corresponding magnetization values can be altered by the change of the base concentration. Results showed that cobalt ferrite nanoparticles, which are synthesized using base concentrations between 0.5 and 8.0 M, are superparamagnetic, and the maximum magnetization value among these nanoparticles is 32.4 emu/g.
References
Sun, S.H., Murray, C.B., Weller, D., Folks, L., Moser, A.: Monodisperse FePt nanoparticles and ferromagnetic FePt Nanocrystal superlattices. Science 287, 1989–1992 (2000)
Hiergeist, R., Andrä, W., Buske, N., Hergt, R., Hilger, I., Richter, U., Keiser, W.: Application of magnetite ferrofluids for hyperthermia. J. Magn. Magn. Mater. 201, 420–422 (1999)
Lee, H., Shao, H.P., Huang, Y.Q., Kwak, B.: Synthesis of MRI contrast agent by coating superparamagnetic iron oxide with chitosan. IEEE Trans. Magn. 41(10), 4102–4104 (2005)
Mornet, S., Vasseur, S., Grasset, F., Veverka, P., Goglio, G., Demourgues, A., Portier, J., Pollert, E., Duguet, E.: Magnetic nanoparticle design for medical applications. Progress in Solid State Chemistry 34, 237–247 (2006)
Jain, T.K., Morales, M.A., Sahoo, S.K., Leslie-Pelecky, D.L., Labhasetwar, V.: Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol. Pharm. 2(3), 194–205 (2005)
Mc Gill, S.L., Cuylear, C.L., Adolphi, N.L., Osińki, M., Symth, H.D.C.: Magnetically responsive nanoparticles for drug delivery applications using low magnetic field strengths. IEEE Trans. Magn. 8(1), 33–42 (2009)
Gyergyek, S., Drofenik, M., Makovec, D.: Oleic acid coated CoFe2O4 nanoparticles synthesized by co-precipitation and hydrothermal synthesis. Mater. Chem. Phys. 133, 515–522 (2012)
Karaagac, O., Kockar, H.: Iron oxide nanoparticles co-precipitated in air environment: Effect of [Fe+2]/[Fe+3] ratio. IEEE Trans. Magn. 48, 1532–1536 (2012)
Karaagac, O., Kockar, H.: Effect of synthesis parameters on the properties of superparamagnetic iron oxide nanoparticles. J. Supercond. Nov. Magn. 25, 2777–2781 (2012)
Kim, Y.I., Kim, D., Lee, C.S.: Synthesis and characterization of CoFe2O4 magnetic nanoparticles prepared by temperature-controlled coprecipitation method. Phys. B 337, 42– 51 (2003)
Zhang, Y., Yang, Z., Yin, D., Liu, Y., Fei, C.L., Xiong, R., Shi, J., Yan, G.L.: Composition and magnetic properties of co-precipitation method. J. Magn. Magn. Mater. 322, 3470–3475 (2010)
Chinnasamy, C.N., Jeyadevan, B., Perales-Perez, O., Shinoda, K., Tohji, K., Kasuya, A.: Growth dominant co-precipitation process to achieve high coercivity at room temperature in CoFe2O4 nanoparticles. IEEE Trans. Magn. 38(5), 2640–2642 (2002)
Pullar, R.C.: Hexagonal ferrites: a review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 57, 1191–1334 (2012)
Cullity, B.D.: Elements of X-ray diffraction. Addison-Wesley, USA (1978)
Kuruva, P., Matteppanavar, S., Srinath, S., Thomas, T.: Size control and magnetic property trends in cobalt ferrite nanoparticles synthesized using an aqueous chemical route. IEEE Trans. Magn. 50, 5200108 (2014)
Senapati, K.K., Borgohain, C., Phukan, P.: Synthesis of highly stable CoFe2O4 nanoparticles and their use as magnetically separable catalyst for Knoevenagel reaction in aqueous medium. J. Mol. Catal. A Chem. 339, 24–31 (2011)
Balavijayalakshmi, J., Suriyanarayanan, N., Jayapraksah, R.: Influence of copper on the magnetic properties of cobalt ferrite nanoparticles. Mater. Lett. 81, 52–54 (2012)
Laurent, S., Forge, D., Port, M., Roch, A., Robic, C., Elst, L.V., Muller, R.N.: Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 108, 2064–2110 (2008)
Jovanovıc, S., Spreitzer, M., Otonicar, M., Jeon, J.H., Suvorov, D.: pH control of magnetic properties in precipitation-hydrothermal-derived CoFe2O4. J. Alloys Compd. 589, 271–277 (2014)
Maaz, K., Mumtaz, A., Hasanain, S.K., Ceylan, A.: Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J. Magn. Magn. Mater. 308(2), 289–295 (2007)
Morales, M.P., Veintemillas-Verdaguer, S., Montero, M.I., Serna, C.J., Roig, A., Casas, Ll., Martinez, B., Sandiumenge, F.: Surface and internal spin canting in γ-Fe2O3 nanoparticles. Chem. Mater 11, 3058–3064 (1999)
Zhang, Y., Yang, Z., Yin, D., Liu, Y., Fei, C.L., Xiong, R., Shi, J., Yan, G.L.: Composition and magnetic properties of cobalt ferrite nano-particles prepared by the co-precipitation method. J. Magn. Magn. Mater. 322, 3470–3475 (2010)
Artus, M., Tahar, L.B., Herbst, F., Smiri, L., Villain, F., Yaacoub, N., Grenèche, J.M., Ammar, S., Fiévet, F.: Size-dependent magnetic properties of CoFe2O4 nanoparticles prepared in polyol. J. Phys. Condens. Matter 23, 506001 (2011)
Kodama, R.H.: Magnetic nanoparticles. J. Magn. Magn. Mater. 200, 359–372 (1999)
Acknowledgments
This work was supported by Balikesir University Research Grant No. BAP 2014/53. Authors would like to thank the State Planning Organization, Turkey, under Grant No. 2005K120170 for VSM system. The authors also thank the Chemistry Department, Balikesir University, Turkey, for XRD and FT-IR measurements and the National Nanotechnology Research Center (UNAM), Bilkent University, Turkey, for TEM analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karaagac, O., Bilir, B. & Kockar, H. Superparamagnetic Cobalt Ferrite Nanoparticles: Effect of Temperature and Base Concentration. J Supercond Nov Magn 28, 1021–1027 (2015). https://doi.org/10.1007/s10948-014-2798-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-014-2798-3