Abstract
A systematic investigation has been carried out to achieve and characterize single-domain cobalt ferrite nanoparticles of high saturation magnetization with least particle size. The samples were obtained by adding different contents of chelating agent, viz., polyvinylpyrrolidone, to cobalt ferrite in the ratios 1:1–3:1 and with careful heat treatment using sol–gel technique. The ferrite samples were annealed in the temperature range 400–1100 °C in the intervals of 100 °C for achieving crystallization to the nanoscale. The prepared samples were characterized by XRD and TEM techniques to identify single-phase spinel structure and the particle size. The magnetic properties, viz., saturation magnetization (Ms), coercivity (Hc) and single-domain nature of the samples, were measured using vibrating sample magnetometer. The obtained results have been discussed in detail as a function of the content of chelating agent. The analysis indicated the developed samples are of single-domain cobalt ferrite nanoparticles with a critical size of 12.8 nm, possess high saturation magnetization (71.9 emu/g) and high coercivity (2744 Oe).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The bulk cobalt ferrite, CoFe2O4, with cubic spinel structure possesses high coercivity, moderate saturation magnetization and remarkable chemical stability with high mechanical hardness [1]. In view of such characteristics, this ferrite finds potential applications, for example, they can be used as isotropic permanent magnets, magnetic fluids, as magnetic recording materials [2], in spintronic applications, etc. [3]. Trukhanov et al. have carried out extensive work on different ferrite materials that possess high saturation magnetization [3,4,5,6].
The bulk ferrites normally require high sintering temperatures for their preparation and to achieve the particle size of the order of microns. With the advent of nanotechnology, it has become possible to produce smaller particles with superior electromagnetic properties. In various biomedical and magnetic recording applications, single-domain magnetic nanoparticles of least particle size (usually less than 10 nm [7]) are desirable for achieving the higher values of saturation magnetization and coercivity. The small grain size of these magnetic nanoparticles inhibits the exchange interaction between neighboring grains. The magnetic materials with such property are useful for the biomedical applications such as hyperthermia [7]. In fact, the particle size, shape and the purity of the nanoparticles decide the magnetic characteristics of ferrite material such as cobalt ferrite. In view of this, a strict control over the reduction of the dimensions of nanoparticles down to the size of the single domain is essential [8]. Moreover, the particle size, in turn, is very sensitive to the method of preparation and subsequent heat treatment conditions of the material.
Several efforts have been made by different researchers to synthesize cobalt ferrite nanoparticles through various chemical methods, viz., co-precipitation [9, 10], the reverse micelle [10], sol–gel [11, 12], solution combustion [13], Sol–gel auto-combustion [14], organic acid precursor route [15], wet chemical route [16], hydrothermal [17], etc. Goh et al. [17] developed single-domain cobalt ferrite nanoparticles of size 33 nm with high saturation magnetization and high coercive force. Similarly, Eshraghi et al. [19] have produced single-domain cobalt ferrite nanoparticles of size 7 nm but with low-saturation magnetization and low coercivity. In Table 1, we have presented a brief summary on the particle size, saturation magnetization and coercivity of cobalt ferrite nanoparticles reported by different researchers.
Thus, it is evident from the literature that particle size has a direct relation with saturation magnetization and coercivity. To be more specific, lower the particle size, the smaller is the magnetization and vice versa. Obtaining high magnetization with least particle size is a challenging task in the field of nanomagnetic materials. Among various methods reported, the sol–gel method seems to be more suitable to obtain smaller particle sizes. The content and the kind of chelating agent and also conditions of annealing are very important in controlling the particle size. Eshraghi et al. [19] have prepared cobalt ferrite using citrate precursor method and have observed a decrease in the particle size with increasing molar ratio of total metal acetates and the citric acid. In the present work, we have chosen sol–gel method for the synthesis of cobalt ferrite nanoparticles. The thorough literature survey indicates virtually no systematic studies are available on cobalt ferrite nanoparticles of size < 10 nm with high saturation magnetization. Motivated by these observations, the present work is aimed to develop single-phase cobalt ferrite with high magnetization and least particle size < 10 nm using sol–gel method.
2 Experimental
Appropriate amounts of AR-grade reagents of ferric nitrate, Fe(NO3)3·9H2O, and cobalt nitrate, Co(NO3)2·6H2O, were used to prepare cobalt ferrite. Polyvinylpyrrolidone (PVP) was used as a chelating agent to control the particle size. Solutions of these materials were prepared by dissolving them separately in a minimum amount of de-ionized water at ambient temperature. Initially, PVP solution was subjected to continuous stirring using an overhead stirrer until a colorless, transparent solution was obtained. The nitrate solutions were added together and the resultant mixture was added to the polymer solution and stirred for 6 h. The obtained admixture is called as precursor. Three combinations of precursor solutions were prepared by varying the amount of PVP with respect to cobalt ferrite in the weight ratio 1:1 (CF-1), 2:1(CF-2), and 3:1(CF-3). The thoroughly mixed precursor was heated at 80 °C in air under continuous stirring for 15 h till a dark brown gel was observed and further dried at 80 °C in air for nearly 10 h and finally orange color powders were obtained.
To understand the influence of the content of chelating agent on the purity of cobalt ferrite phase and particle size, the prepared powders were annealed at 300 °C, 400 °C, 600 °C, 700 °C, and 800 °C with a typical heating rate of 5 °C per minute using a programmable muffle furnace for 1 h and cooled down to room temperature gradually.
The annealed samples were characterized by X-ray diffraction (XRD), FTIR and transmission electron microscopy (TEM) techniques. X-ray diffraction patterns were recorded using CuKα (1.5406 Å) radiation in the range 2θ from 15° to 80°. Infrared spectra were recorded in the spectral region 400–3000 cm− 1 using a Hyperion microscope with vertex 80 FTIR spectrophotometer up to a resolution of 0.2 cm− 1.Transmission electron microscopy studies were carried out using Tecnai G2 20 S-Twin transmission electron microscope (TEM) with 200-kV electron beam. The particle size distribution was evaluated using the obtained TEM photographs. Magnetic characterization of the cobalt ferrite nanoparticles was performed using vibrating sample magnetometer (Lakeshore 4700VSM) at ambient temperature.
3 Results and discussion
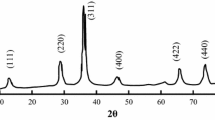
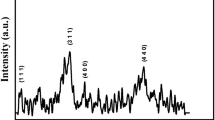
Figure 1 shows X-ray diffraction patterns of CF-1 (a), CF-2 (b), and CF-3 (c) samples, annealed at 300 °C. Diffraction peaks due to reflection from different planes of cobalt ferrite along with impurity phases were identified in the patterns of CF-1 and CF-2 samples. The peaks in the diffraction pattern of CF-2 are observed to be relatively broader with feeble intensity when compared with those of CF-1. However, in pattern of the sample CF-3 (annealed at same temperature) well-resolved diffraction peaks could not be observed. It may be due to the presence of large amount of chelating agent in this sample which might have influenced the chemical reaction between the ingredients of the sample.
To have a clear understanding over crystalline structure, the fresh samples were annealed at 400 °C and XRD patterns were recorded; the obtained diffractograms are presented in Fig. 2. The patterns of these samples exhibited sharper and intense peaks when compared to those of the samples heat treated at 300 °C. Some additional feeble peaks were also observed in the patterns of CF-1 and CF-2 samples due to annealing at 400 °C. Moreover, the diffractogram of CF-3 sample annealed at this temperature exhibited well-resolved peaks due to the reflections from different planes of cobalt ferrite spinel structure.
Since the well-resolved diffraction peaks could not be detected in the diffractograms of the samples CF-1 and CF-2 annealed up to 400 °C, these samples were annealed at still elevated temperatures, viz., 600 °C, 700 °C and 800 °C, under similar conditions. The obtained patterns of these samples are presented in Figs. 3 and 4, respectively. Even after annealing at 800 °C, the pattern still exhibited diffraction peaks due to impurity phases. These impurity peaks were identified as being due to reflections from different planes of α-Fe2O3 and Co3O4 phases [14]. The results of these studies indicate that if one wants to obtain single-phase cobalt ferrite through sol–gel method with low content of chelating agent, the samples are needed to be annealed at higher temperatures, which is a hindrance for achieving particles with smaller dimensions. In view of this, we could come to the conclusion that CF-3 is an ideal combination for obtaining pure cobalt ferrite phase. With this expectation, further work, in the direction of producing cobalt ferrite particles that exhibit high saturation magnetization and with lower particle size (which are desirable for the use of these samples in biomedical and magnetic recording applications), has been carried out with CF-3 sample.
To obtain further support regarding the formation of cobalt ferrite phase as well as the presence of PVP matrix in the samples, we have recorded FTIR spectra for these samples. Figure 5 shows FTIR spectra of the sample CF-3 recorded after annealing at 300 °C, 400 °C, and 600 °C. The spectra exhibited Fe–O vibrational band (octahedral band, Oh) [11, 12, 23] at about 590 cm− 1 in addition to multiple vibrational bands (stretching-modes) in the spectral region 1200‒3000 cm− 1 predicted due to O–H, C–O and C=H groups present in PVP source [24]. With increase of annealing temperature from 300 to 600 °C, the intensity of bands due to these hydroxyl and carbonate groups is gradually diminished and virtually disappeared in the spectrum of the sample annealed at 600 °C, whereas the presence of octahedral vibrational band of Fe ions as become more visible with increasing intensity.
For obtaining quantitative information on the size of the cobalt ferrite particles and its dependence with annealing temperature, we have recorded XRD patterns of the CF-3 samples annealed at different temperatures (500–1100 °C) and presented in Fig. 6.The size of the crystallite has been estimated from Williamson–Hall (W–H) plots [25] using the equation: \(\beta \cos \theta =\frac{{0.9~\lambda }}{D}+4\varepsilon \sin \theta\), where β is the corrected peak broadening \(~\left( {=\sqrt {{\beta _{{\text{measured}}}}^{2} - {\beta _{{\text{instrumental}}}}^{2}} } \right)\), λ (1.5406 Å) the wavelength of X-rays used and θ is the peak position and \(\varepsilon\) represents internal strain. The average crystallite sizes evaluated from the Williamson–Hall plots (Fig. 7) were found to be in the range 8.3–38.6 nm for the samples annealed at different temperatures (Table 2).The lattice constant is estimated from the diffraction peak of each plane and its variation is plotted with Nelson–Riley function F(θ) = (Cos2θ/2Sinθ) + (Cos2θ/2θ) so as to obtain the accurate value of lattice constant for studied samples annealed at different temperatures (Fig. 8). The extrapolation of the obtained graphs to the Y-axis, is predicted to give precise value of lattice constant [10]. The value of lattice constant (of the cubic-structured cobalt ferrite samples) obtained from these plots is estimated to be ~ 8.38 Å; this value is observed to be near invariant with annealing temperature and further found to be in good agreement with that of reported value [11].
Figure 9 shows transmission electron micrographs and normal distribution of the particle size of CF-3 annealed at different temperatures. All the histograms obtained from TEM micrographs for evaluating the average particle diameter can be well fitted by a Gaussian distribution by considering not less than 100 particles in each case. Nanoparticles with an average particle diameter ranging from 7.8 to 13.1 nm are evidenced from these photographs and the values are observed to be nearer to the crystallite sizes obtained from Williamson–Hall plots (Table 3).
4 Magnetic properties
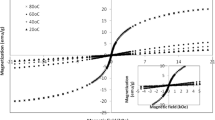
Table 2 shows the room temperature magnetic parameters of CF-1, CF-2 and CF-3 samples annealed at 400, 600, 700 and 800 °C. It was observed that the magnetization is increasing with increasing annealing temperature and also with content of chelating agent. The lower values of the magnetization exhibited by the samples CF-1 and CF-2 may be ascribed to the presence of impure oxide phase [26, 27]. Figure 10 shows the hysteresis curves (recorded at room temperature) of CF-3 sample annealed at different temperatures.
Normally, the magnitude of saturation magnetization of cobalt ferrite is dependent on various factors such as the method of preparation, the annealing temperature and the rate of heating. For example, Naseri et al. [12] have developed cobalt ferrite nanoparticles of least size (12.5–29 nm) through PVP-assisted thermal treatment method (also known as sol–gel method) and measured Ms; the obtained value Ms for these samples is found to be very low (1.15–18.02 emu/g) than the generally reported values [11]. These authors have attributed the observed low-value magnetization is mainly due to high heating rate (10 °C/min) of the samples during annealing process. Sangmanee et al. [28] showed that saturation magnetization for this ferrite increases from 9.7 to 56.5 emu/g with decreasing the heating rate from 20 °C/min to 5 °C/min during annealing process. In the current study, we have adopted similar PVP-assisted sol–gel method to obtain cobalt ferrite nanoparticles by annealing at various temperatures with heating rate of 5 °C/min; these samples with the lowest particle size exhibited relatively high saturation magnetization ~ 71.9 emu/g.
Figure 11 represents the variation of saturation magnetization (Μs) and coercivity (Hc) with crystallite size of the sample CF-3. The saturation magnetization exhibited increasing trend with the increase of annealing temperature. The coercivity is observed to increase from 1411 to 2744 Oe with the increase of annealing temperature up to 700 °C and at still higher annealing temperatures, it is observed to decrease. This observation suggests that up to 700 °C of annealing temperature, the material is in the single-domain region and beyond this temperature the material is transformed into multi-domain state [16]. On the other hand, for the samples CF-1 and CF-2 this critical temperature (at where single domain transforms into multi-domain) is observed to be shifted to lower temperature (Table 2). Overall, comparison of these results suggests that with low contents of chelating agents, there is only remote possibility for obtaining single phase with single domain. In other words, this observation clearly demonstrated that the content of chelating is also one of the crucial parameters for obtaining single-domain nanoparticles through sol–gel method. The size of the crystallite at this critical point (the transition point from single domain to multi-domain) is known as critical size and it is identified as 12 nm (Fig. 11) for the CF-3 sample annealed at 700 °C. The corresponding saturation magnetization and the coercivity are found to be 71.9 emu/g and 2744 Oe, respectively. It may be worth mentioning here that even though the sample exhibited higher value of Μs (Table 3) beyond the critical size of the crystallite it is in the multi-domain state. This may be a hindrance for the practical utility of the sample for biomedical applications. Even though it is a general expectation that ferrite materials with the particles with size less than 10 nm must be super-paramagnetic, in the present investigation, the super-paramagnetic behavior could not be visualized. However, for the cobalt ferrite, the minimum size required for the particles to act as super-paramagnetic is reported to 2–4 nm [10, 29] at room temperature. We have also evaluated the magneto-crystalline anisotropy constant, K, using coercivity, Hc, and magnetization, Ms, from Stoner–Wohlfarth relation, Hc = 2K/Ms and it is presented in Table 3; the anisotropy constant, K, and coercivity, Hc, exhibited non-monotonic behavior with particle size.
Overall, in the present study we have been fairly successful, in identifying the optimum content of chelating agent and annealing temperature in obtaining pure single-domain cobalt ferrite nanoparticles with high saturation magnetization with the lowest particle size.
5 Conclusions
The cobalt ferrite with the lowest nanoparticle size with no impurity phases could be made possible by selecting the optimum values of content of chelating agent and annealing temperature. The present study also indicated that the use of smaller amounts of chelating agents creates larger possibility for the presence of undesired impurity oxide phases such as α-Fe2O3 and Co3O4 even at the elevated annealing temperatures.
The sample CF-3 (developed with optimum quantity of chelating agent) exhibited single phase with single domain at lower annealing temperature. The saturation magnetization and remnant magnetization have been observed to be dependent on particle size. The single-domain cobalt ferrite annealed at 700 °C has shown saturation magnetization of 71.9 emu/g and high coercive field of 2744 Oe for the average particle size of 12 nm. The magnetic measurements suggested that the present adopted method is suitable for obtaining single-domain cobalt ferrites with high magnetization and smaller particle size which are desirable parameters for practical applications of these materials.
Further, this material possesses large spontaneous polarization even at room temperature and hence can be considered as suitable for the applications in spintronics.
References
J.G. Lee, J.Y. Park, C.S. Kim, Growth of ultra-fine cobalt ferrite particles by a sol-Gel method and their magnetic properties. J. Mater. Sci. 33, 3965–3968 (1998)
M. Grigorova, H.J. Blythe, V. Blaskov, V. Rusanov, V. Petkov, V. Masheva, D. Nihtianova, Ll.M. Martinez, J.S. Munoz, M. Mikhov, Magnetic properties and Mossbauer spectra of nanosized CoFe2O4powders. J.Magn. Magn. Mater. 183, 163–172 (1998)
S.V. Trukhanov, A.V. Trukhanov, V.A. Turchenko, V.G. Kostishin, L.V. Panina, I.S. Kazakevich, A.M. Balagurov, Crystal structure and magnetic properties of the BaFe12 – xInxO19 (x = 0.1–1.2) solid solutions. J. Magn. Magn. Mater. 417, 130–136 (2016)
A.V. Trukhanov, S.V. Trukhanov, L.V. Panina, V.G. Kostishyn, D.N. Chitanov, I.S. Kazakevich, A.V. Trukhanov, V.A. Turchenko, Strong corelation between magnetic and electrical subsystems in diamagnetically substituted hexaferrites ceramics. Ceram. Int. 43, 5635–5641 (2017)
S.V. Trukhanova, A.V. Trukhanova, V.G. Kostishyn, L.V. Panina, V.A. Turchenko, I.S. Kazakevich, An.V. Trukhanova, E.L. Trukhanov, V.O. Natarov, A.M. Balagurov, Thermal evolution of exchange interactions in lightly doped barium hexaferrites. J. Magn. Magn. Mater. 426, 554–562 (2017)
S.V. Trukhanov, A.V. Trukhanov, V.A. Turchenko, An.V. Trukhanov, E.L. Trukhanova, D.I. Tishkevich, V.M. Ivanov, T.I. Zubar, M. Salem, V.G. Kostishyn, L.V. Panina, D.A. Vinnik, S.A. Gudkova, Polarization origin and iron positions in indium doped barium hexaferrites. Ceram. Int. 44, 290–300 (2018)
E.S. Murdock, R.F. Simmons, R. Davidson, Roadmap for 10 Gbit/in2 media: challenges. IEEE Trans. Magn. 28, 3078–3083 (1992)
C.J. O’Connor, V. Kolesnichenko, E. Carpenter, C. Sangregorio, W. Zhou, A. Kumbhar, J. Sims, F. Agnoli, Fabrication and properties of magnetic particles with nanometer dimensions. Synth. Met. 122, 547–557 (2001)
Y. Zhang, Z. Yang, D. Yin, Y. Liu, C. Fei, R. Xiong, J. Shi, G. Yan, Composition and magnetic properties of cobalt ferrite nanoparticles prepared by the co-precipitation method. J. Magn. Magn. Mater. 322, 3470–3475 (2010)
I. Sharifi, H. Shokrollahi, M.M. Doroodmand, R. Safi, Magnetic and structural studies on CoFe2O4 nanoparticles synthesized by co-precipitation, normal micelles and reverse micelles methods. J. Magn. Magn. Mater. 324, 1854–1861 (2012)
K.S. Rao, G.S. Choudary, K.H. Rao, C. Sujatha, Structural and magnetic properties of ultrafine CoFe2O4 nanoparticles. Procedia Mater. Sci 10, 19–27 (2015)
M.G. Naseri, E.B. Saion, H.A. Ahangar, A.H. Shaari, M. Hashim, Simple synthesis and characterization of cobalt ferrite nanoparticles by a thermal treatment method. J. Nanomater 2010, 1167–1174 (2010)
D.M. Jnaneshwara, D.N. Avadhani, B.D. Prasad, B.M. Nagabhushana, H. Nagabhushana, S.C. Sharma, C. Shivakumara, J.L. Rao, N.O. Gopal, S.C. Ke, Chakradhar, Electron paramagnetic resonance, magnetic and electrical properties of CoFe2O4 nanoparticles. J. Magn. Magn. Mater. 339, 40–45 (2013)
P.S. Aghav, V.N. Dhage, M.L. Mane, D.R. Shengule, R.G. Dorik, K.M. Jadhav, Effect of aluminum substitution on the structural and magnetic properties of cobalt ferrite synthesized by sol–gel auto combustion process. Phys. B 406, 4350–4354 (2011)
R.M. Mohamed, M.M. Rashad, F.A. Haraz, W. Sigmund, Structure and magnetic properties of nanocrystalline cobalt ferrite powders synthesized using organic acid precursor method. J. Magn. Magn. Mater 322, 2058–2064 (2010)
K. Maaz, A. Mumtaz, S.K. Hasanain, A. Ceylan, Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J. Magn. Magn. Mater. 308, 289–295 (2007)
S.C. Goh, C.H. Chia, S. Zakaria, M. Yusoff, C.Y. Haw,, N.M. Huang, H.N. Lim, Sh. Ahmadi, Hydrothermal preparation of high saturation magnetization and coercivity cobalt ferrite nanocrystals without subsequent calcinations. Mater. Chem. Phys. 120, 31–35 (2010)
M. Eshraghi, P. Kameli, Magnetic properties of CoFe2O4 nanoparticles prepared by thermal treatment of ball-milled precursors. Curr. Appl. Phys. 11, 476–481 (2011)
A. Mumtaz, K. Maaz, B. Janjua, S.K. Hasanain, M.F. Bertino, Exchange bias and vertical shift in CoFe2O4 nanoparticles. J. Magn. Magn. Mater. 313, 266–272 (2007)
V. Pillai, D.O. Shah, Synthesis of high-coercivity cobalt ferrite particles using water-in-oil microemulsions. J. Magn. Magn. Mater. 163, 243–248 (1996)
C.N. Chinnasamy, M. Senoue, B. Jeyadevan, O. Perales-Perez, K. Shinoda, K. Tohji, Synthesis of size-controlled cobalt ferrite particles with high coercivity and squareness ratio. J. Colloid Interface Sci. 263, 80–83 (2003)
V. Kumar, A. Rana, M.S. Yadav, R.P. Pant, Size-induced effect on nano-crystalline CoFe2O4. J. Magn. Magn. Mater. 320, 1729–1734 (2008)
P. Sobhanachalam, C.V. Kumari, G. Sahaya Baskaran, P. Syam Prasad, N. Veeraiah, V. Ravi Kumar, On identifying efficient modifier oxide in improving bioactivity of Fe2O3 doped calcium oxy fluoroborophosphate glasses. J. Alloys Compd. 692, 219–226 (2017)
Y.J. Kwon, K.H. Kim, C.S. Lim, K.B. Shim, Characterization of ZnO nanopowders synthesized by the polymerized complex method via an organochemical route. J. Ceram. Process. Res. 3, 146–149 (2002)
K. Venkateswarlu, A. Chandra Bose, N. Rameshbabu, X-ray peak broadening studies of nanocrystalline hydroxyapatite by Williamson–Hall analysis. Phys. B 405, 4256–4261 (2010)
P. Laokul, V. Amornkitbamrung, S. Seraphin, S. Maensiri, Characterization and magnetic properties of nanocrystalline CuFe2O4, NiFe2O4, ZnFe2O4 powders prepared by the Aloe vera extract solution. Curr. Appl. Phys. 11, 101–108 (2011)
Y. Kinemuchi, K. Ishizaka, H. Suematsu, W. Jiang, K. Yatsui, Magneticproperties of nanosize NiFe2O4 particles synthesized by pulsed wire discharge. Thin Solid Films 407, 109–113 (2002)
M. Sangmanee, S. Maensiri, Nanostructures and magnetic properties of cobalt ferrite (CoFe2O4) fabricated by electrospinning. Appl. Phys. A 97, 167–177 (2009)
K. Srinivasa Rao, S.V. RangaNayakulu, M. Chaitanya Varma, G.S.V.R.K. Choudary, K.H. Rao, Controlled phase evolution and the occurrence of single domain CoFe2O4 nanoparticles synthesized by PVA assisted sol-gel method. J. Magn. Magn. Mater. 451, 602–608 (2018)
Acknowledgements
We are thankful to Dept. of ACMS, IIT Kanpur, for providing magnetic measurements, SAIF, IIT Bombay, for providing the facility to record FTIR spectra, Dept. of Metallurgical and Materials Engineering, RGUKT, IIIT Nuzvid, for utilizing XRD facility and also thankful to the IIC, IIT Roorkee, for providing TEM images.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patta, G.R., Kumar, V.R., Rao, K.H. et al. Synthesis and studies on magnetic properties of single-phase cobalt ferrite nanoparticles: influence of content of chelating agent. Appl. Phys. A 125, 187 (2019). https://doi.org/10.1007/s00339-019-2489-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-019-2489-9