Abstract
In this study, nano-crystalline ferrite powders with the composition of Ni0.5Zn0.5GdxFe2−xO4 with the values of x ranging x = 0.00, 0.05, 0.10 were prepared through citrate gel auto-combustion method. The prepared samples were characterized by X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR). By increasing the composition of Gd, we observed that lattice parameter increases. From XRD, we observed that crystallite size is maximum at x = 0.10 and strain is negative. From FTIR, we observed that force constants were found to vary with sintering temperature and reporting a cation redistribution and modification in the spinel ferrite unit cell.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Large surface area and quantum size effect of magnetic nano-particles change some of magnetic properties and exhibit ferromagnetic behavior. Ferrite are magnetic oxide which contain iron oxide as a major component and have enormous applications in electronic industry [1, 2] such as multi-layer chip inductor, electromagnetic interference, and gas sensor’s. The structural and the magnetic properties of Ni–Zn ferrite wear changes with the preparation method. There are several preparation methods which have been employed in nano-ferrite, co-precipitation method [3], combustion synthesis [4], microwave sintering [5], hydrothermal processing, [6], and sol–gel method [7]. Conventional soiled state ceramic process [7]. This technique relates high temperature and suffers various defects. For the preparation of nano-ferrite, sol–gel method is the best [7,8,9]. Ferrites are prepared by using citrate gel auto-combustion method [7, 10, 11]. The advantage of this method is the particle size should be in nano-size. In the present investigation, nano-particles of Gd substituted Ni–Zn ferrite structural properties studied.
In Ni–Zn, ferrite possesses a mixed spinel structure in which Ni ions occupy octahedral sites (B), Zn ions occupy tetrahedral sites, and Fe3+ ions occupy A and B sites. The distribution of cations over tetrahedral and octahedral sites, the structural properties are known to depend not only on the composition but also in the cation distribution.
2 Experimental
Ni0.5Zn0.5GdxFe2−xO4 with the values of x ranging x = 0.00, 0.05, 0.10 was prepared through citrate gel auto-combustion method. In this preparation, analytical grade Ni nitrate, Zn nitrate, iron nitrate, and Gd nitrate were used in proper stoichiometric ratios. All the nitrates were stirred separately on molecular state for one hour. Now, the citric acid is added to mixed state and the temperature rises gradually. The solution slowly transformed into the gel, and the thickness of the gel continuously increases and becomes dense [12]. Finally it burnt and gives rise to brown color was formed. By using agate mortar, this powder was grounded for one hour to get fine-grounded ferrite powder. The XRD data were recorded at room temperature using Cu–Kα (λ = 1.5406 Å) radiation for all the samples. By using KBr pellets, FTIR (300–1000 cm−1) data of samples were recorded by infrared radiation spectrophotometer [13].
3 Results and Discussion
3.1 X-ray Analysis
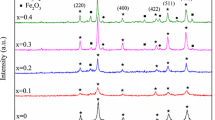
The XRD patterns for the samples Ni0.5Zn0.5GdxFe2−xO4, where x = 0, 0.05, 0.1, 0.15, are shown in Fig. 44.1. It confirms that the single-phase spinel cube and all the peaks observed match well with Ni–Zn ferrites reported earlier [14]. Table 44.1 shows particle size, lattice constant, and strain. Figure 44.1 shows XRD patterns of the prepared samples. The average particle size is determined using Scherrer’s formula [15]. The lattice parameter increases from x = 0.00–0.15, but particle size decreases up to 0.10 and increases at 0.15 due to strain in the sample. Except x = 0.0, all samples are having negative strain. The lattice parameter ‘a’ is determined by the following relation:
Where ‘d’ is interplanar spacing which can be determined by using Bragg’s law for plane (hkl).
For exact calculation, the lattice parameter for each sample Nelson-Riley method was used
IR spectra analysis of prepared samples is shown in Fig. 44.2 in the range of 300–1000 cm−1. The force constants for the bands corresponding to O–H stretching vibration decrease with the increase of Gd concentration [16]. This decrease is due to the loss of the residual water in the samples.
The force constants for ‘A’ site and ‘B’ site ‘Kt’ and ‘K0’, respectively, are calculate by using Waldron relation.
where M1 and M2 are molecular weights of cations on ‘A’ site and ‘B’, respectively (Fig. 44.3, Table 44.2).
4 Conclusions
Samples of nano-crystalline Ni0.5Zn0.5GdxFe2−xO4 have been successfully synthesized using citrate gel method. It is observed from XRD analysis that particle size, lattice parameter, and strain. From FTIR analysis, it is observed that O–H stretching vibration band intensities decreases with increase of Gd concentration.
References
Modak, S., Ammar, M., Mazaleyrat, F., Das, S., Chakrabarthi, P.K.: J. Alloys Compd. 473, 15 (2009)
Nakamura, N.: J. Appl. Phys. 88, 348 (2000)
Hakim, M.A., Saha, D.K., Kibria, A.K.M.F.: Bang. J. Phys. 3, 57 (2007)
Yue, Z., Zhou, J., Gui, Z.: J. Magn. Magn. Mater. 233, 224 (2001)
Bhaskar, A., Kanth, B.R., Murthy, S.R.: J. Magn. Magn. Mater. 283, 109 (2004)
Li, X., Wang, G.: J. Magn. Magn. Mater. 321, 1276 (2009)
Shinde, T.J., Gadkari, A.B., Vasambekar, P.N.: J. Magn. Magn. Mater. 333, 152 (2013)
Prasad, M.S.R., Prasad, B.B.V.S.V., Rajesh, B., Rao, K.H., Ramesh, K.V.: J. Magn. Magn. Mater. 323, 2115 (2011)
Eltabeyetal, M.M., Agami, W.R., Mohsen, H.T.: J. Adv. Res. 601 (2014)
Nasir, S., Asghar, G., Malik, M.A., Anis-ur-Rehman, M.: J. Sol-Gel. Technol 59, 111 (2011)
Verma, A., Goel, T.C., Mendiratta, R.G., Gupta, R.G.: J. Magn. Magn. Mater. 192, 271 (1999)
Prasad, M.S.R., Babu, B.R., Ramesh, K.V., Trinath, K.: J. Supercond. Nov. Magn. 27, 2735 (2014)
Kosoeoglu, Y., Oleiwi, M.I.O., Yilgin, R., Koebay, A.N.: Cerma. Int. 38, 6671 (2012)
Cullity, B.D., Stock, S.R.: Principles of X-ray Diffraction, 3rd edn. Prentice Hall Inc. (2001)
Klug, H.P., Alexender, L.E.: X-ray diffraction procedures for polycrystalline and amorphous materials, p. 637. Newyork, Wiley (1997)
Maensiri, S., Masingboon, C., Poncho, B., Seraphin, S.: Scripta Mater. 56,797–800 (2007)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Srikanth, M., Nagesh, G.V., Ramesh, K.V. (2019). Structural Studies on the Trivalent Gadolinium Substituted Ni–Zn Ferrite System. In: Pujari, S., Srikiran, S., Subramonian, S. (eds) Recent Advances in Material Sciences . Lecture Notes on Multidisciplinary Industrial Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-13-7643-6_44
Download citation
DOI: https://doi.org/10.1007/978-981-13-7643-6_44
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7642-9
Online ISBN: 978-981-13-7643-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)