Abstract
The influence of zirconium (Zr) addition to the Bi2Sr2CaCu2O8+δ (Bi2212) system on its phase formation, microstructure, transport, and magnetic properties is investigated. Samples have been characterized by means of X-ray diffraction analysis (XRD), scanning electron microscopy equipped with energy dispersive of X-ray analysis (SEM/EDX), and resistivity versus temperature measurement. The results show an increase of the critical temperature of superconductive transition T c for x=0.01 and in all the samples containing Zr the Bi2212 phase is the majority. SEM observations show whiskers grains randomly distributed and microstructural change due to the addition of Zr. Resistivity measurement show that the temperature of the onset of transition T c increases by 9 K for x=0.005, 0.02, and 5 K for x=0.01 of ZrO2. An improvement of the normal state conductivity is obtained for the lowest content of Zr (x=0.005). In the normal state, all the samples exhibit a metallic behavior except the sample with x=0.01, which exhibit a semiconductor character.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The high-temperature superconductors have two major drawbacks: they are brittle, and their critical current density is very low. They are not suitable for making wires and tapes. Yttrium-based superconductors are very sensitive to humidity; Tl and Hg-based superconductors are not suitable for safe handling and for the industrial applications despite their high T c . Different phases of the Bi-based superconductors can be formed having different composition, structure, and properties. In fact, it is well known that the Bi-based superconductors consist of at least three phases: Bi2Sr2CuO6+γ (Bi2201), Bi2Sr2CaCu2O8+δ (Bi2212), and Bi2Sr2Ca2Cu3O10 (Bi2223). The thermodynamical stability limits between them have been investigated in polycrystalline samples of macroscopic sizes [1] and this information has been exploited in order to design the most suitable processes for the synthesis of pure samples in the different phases. The critical current density (J c ) and critical temperature (T c ) are the parameters of primary importance for potential applications of HTSC and the J c value is primarily limited due to the insufficient flux pinning properties. A number of particularly effective artificial pinning centers have been tested for superconductors, such as AgO2, MgO, SiC, and SrSO4 [2], to improve both the electrical and mechanical properties [3]. In the case of (Bi, Pb) Sr–Ca–Cu–O (2223) single crystal or polycrystalline, added by Zr, many reports show the increase of the critical temperature T c and the critical current density under magnetic fields [4]. In order to enhance the critical current density of the BSCCO system, the number of pinning centers should be increased. The effect of nanosize Al2O3, ZrO2 additions on the microstructure and pinning properties of polycrystalline (Bi, Pb)-2223 was analyzed. The Al2O3, ZrO2 content in the sample varied from 0.0 to 1.0 wt% of the total mass of the sample. Up to 0.1 wt% leads to an increase of J c and T c values [5, 6].
Addition of perovskites SrAO3 (A=Zr, Sr, etc.) in the Bi-2212 matrix shows the partial incorporation of the particles into Bi-2212 grains and partially agglomerated, forming thin closed areas between the Bi-2212 lamellas, which results in the increase of J c and the decrease of T c .
Additives can be used as effective pinning centers in BSCCO superconductors due to the size of its coherence length in the range of nanometers. The superconducting order parameter may be depressed locally by inhomogeneities in stoichiometry even at a single atomic site, the coherence length being short in the HTSC. This situation is verified by the vacancies of oxygen in CuO2 planes, which may act as pinning centers [4]. The superconducting (T c , J c ) and structural properties can be modified by substitution or addition of elements, with changing ionic radius and bonding characteristics, leading to enhance or depress locally the oxygen concentration. Additional holes or electrons must be taken in account for the effects of additives on the variation of the superconducting properties.
In this report, we present a study of the influence of ZrO2 additions in the Bi2212 superconducting phase. Based on previous results we are assuming that Zr ion can substitute Cu ions in the Bi2212 phase. The dopant can influence the kinetics and mechanism of HTSC phase formation, thus changing the final microstructure of the superconductor.
2 Experiments
The standard solid state reaction method was used to obtain the nominal composition 2212. This method has involved the use of Bi2O3, SrCO3, CaCO3, and CuO powders. Initially, after weighting, powders were mixed and ground to obtain a homogenous mixture. These mixtures were heated twice at 800 °C for 20 h in an alumina crucible to decompose carbonates. The calcined powders were ground again in a mortar and were pressed in a pellet shape under pressure of 5 ton/cm3. These pellets were sintered at 840 °C, reached at 5 °C/min, for 60 h in atmosphere. ZrO2 powders was added to the obtained precursors with yielding the following components Bi2Sr2CaCu2Zr x O8+δ (x=0, 0.005, 0.01, 0.02, 0.05). The powders were mixed, reground, and pressed again. The sintering treatments of the pellets were performed in air at 860 °C for 60 h twice with intermediate grinding. XRD spectra of the samples were recorded using a Jeol Rigala Multiflex diffractometer with Cu K α radiation (λ=1.5418 Å) and incident angle in the range 2θ=3–60∘. The SEM micrographs were taken on a JEOL 6390-LV microscope. Analysis of atomic sample composition has been made by means of EDX. The DC resistivity versus temperature of samples has been measured using a standard four point probe method.
3 Results and Discussions
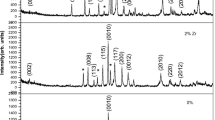
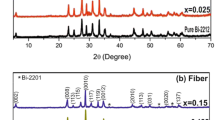
Figure 1 shows the XRD patterns of the samples after the last stage of heat treatment. These patterns show that the Bi2212 phase exists as a majority phase. The intensity I max of the main peak of Bi2212 increases for 1 % and 5 % of Zr. This means that the addition of Zr promotes the formation of the Bi2212 phase. In the sample added by 5 % of Zr, the peak at 2θ=34∘ indicates the presence of the CuO parasitic phase. This may reflect oxygen depletion in the sample during heat treatment or the presence of small oxygen content inhomogeneities. Impurity phases are a consequence of an unbalance of the stoichiometry ratio and insufficient synthesis time [7]. No secondary phase containing Zr ions is observed at this stage. This shows that the Zr4+ ions may enter the crystal lattice of Bi2212.
The XRD patterns have been indexed to a tetragonal unit cell. The variations of the lattice parameters versus the content of the doping element are shown in Table 1. The lattice parameters were calculated from the XRD data. The decrease in c-axis values with increasing concentration of Zr is attributed to the smaller ionic radius of Zr+4 compared to the ionic radius of Cu+2 and effect of its larger valence. This latter gives rise to a stronger ionic bonding to the excess oxygen in the BiO planes, causing a tighter binding, and hence reducing the c-axis lattice constant. Increase in the lattice parameter a may be attributed to the change in the Cu–O bond distance.
SEM micrographs of the samples are presented in Fig. 2. In a pure sample, a plate-like nature of grains, typical of the HTSC Bi based superconducting phase, may be observed in the figure. The average size of the grain is estimated to be less than 3 μm. The morphology of the grains changes with Zr content (0–5 %). The sample added with 1 % Zr shows a microstructure with highly dense, aligned, and much larger grains. The sample with x=0.02 has grains more ordered and with smaller size, which provide stronger contact between them, than in the other samples. Enhancement of the Zr content leads to an important increase of grain size as it is observed in sample with x=0.05. White areas may be observed in the samples doped with 2 % and 5 % of Zr. They are larger in the sample doped with 5 % of Zr than in the one doped with 2 % and may indicate the presence of Zr.
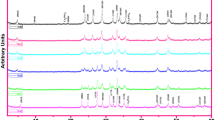
Figure 3 presents the EDX spectrum of the surface of the sample doped with 0 %, 1 %, 2 %, and 5 % of zirconium. The EDX plot reveals no extra peaks and reflects the presence of all the constituents. All the peaks are related to Bi, Sr, Ca, Cu, Zr, and O atoms.
These results indicate the incorporation of Zr into the Bi2212 lattice and reveal that the elemental composition of all the samples does not contain any foreign element.
The resistivity versus temperature measurements of the samples are shown in Fig. 4. Except for the one containing x=0.01 of added Zr, all the samples show in the normal state a metallic behavior with temperature. Except for the sample containing 5 % of Zr, an enhancement of \(T_{c}^{\mathrm{onset}}\) is observed and may be related to a better oxygenation of the grains. The sample added with 0.005 of Zr shows an improvement of the conductivity in the normal state revealed by the lowest value of the resistivity in the normal state suggesting an occurrence of optimally or even over doped regime. For all the other samples, the resistivity in the normal state is higher than in the undoped sample and increases with the content of Zr. The sample added with 0.02 of Zr presents, in its resistivity versus temperature curve, a double transition suggesting the presence of the 2223 phase. The critical temperature has been improved by about 5 to 9 K for the rate below or equal 2 %.
The enlarged transition width of the doped samples may be attributed to the impurities existing in the samples. The values for all the samples of the zero-resistance transition temperature \(T_{c_{0}}\) and of the onset of the transition \(T_{c}^{\mathrm{onset}}\) are reported in Table 2. Table 2 shows that the pure sample has a \(T_{c} ^{\mathrm{onset}}\) of 82 K while the doped samples with x=0.005, 0.01, and 0.02 of Zr have their one above 85 K.
For the low values of x (0.005 and 0.01), \(T_{c_{0}}\) is higher than the undoped sample one, but degrades more for the higher content of Zr. This suggests that low content of Zr improves the connectivity of the grains, and consequently lowers the intergranular resistivity. Higher content gives an opposite scenario lowering \(T_{c_{0}}\) and practically doubling the transition width. The large increase of the grain size observed for the highest content of Zr is then obtained with a degradation of the grain boundaries. Thus high content of Zr can lead to an increase of the weak-links [8]. This behavior can be also explained, when high rate is used, by the substitution of the spinless Zr+4 ion in the Cu+2 site. The perturbation of the local magnetic ordering can destroy the coherence of the phase φ of the order parameter ψ [9, 10], and results in pair breaking effect [11, 12]. Thus, at low content, the ionic radius and the valence are the parameters of control. At higher content, many factors act in the same time to degrade the superconducting properties: grain connectivity, spinless substitution, and lowering of the c-axis parameter suggesting a change of the coupling between CuO2 planes.
4 Conclusion
ZrO2 addition on the Bi2212 phase is a way to improve its superconducting properties when low content is used. Structural analysis shows that Zr substitutes on the Cu site, which results when a high rate is used in a dramatic degradation of the zero resistance temperature of transition and a large increase of the transition width.
At a rate lower or equal to 1 %, the T c is improved and the transition width is reduced.
References
Tinkham, M.: Introduction to Superconductivity. McGraw-Hill, New York (2004)
Jia, Z.Y., Tang, H., Yang, Z.Q., Xing, Y.T., Wang, Y.Z., Qiao, G.W.: Physica C 337, 130 (2000)
Xu, K.-X., Qiu, J.-H., Shi, L.-Y.: Supercond. Sci. Technol. 19, 178 (2006)
Biju, A., Aloysius, R.P., Syamaprasad, U.: Supercond. Sci. Technol. 18, 1454 (2005)
Biju, A., Sarun, P.M., Aloysius, R.P., Syamaprasad, U.: Supercond. Sci. Technol. 19, 1023 (2006)
Sasakura, H., Miura, O., Ito, D.: Physica C 357–360, 216 (2001)
Sekkina, M.M.A., Elsabawy, K.M.: Physica C 377, 254 (2002)
Nkum, R.K., Datars, W.R.: Supercond. Sci. Technol. 8, 822 (1995)
Dynes, R.C., White, A.E., Graybeal, J.M., Garno, J.P.: Phys. Rev. Lett. 57, 2195 (1986)
Graybeal, J.M., Beasley, M.A.: Phys. Rev. B 29, 4167 (1984)
White, A.E., Dynes, R.C., Garno, J.P.: Phys. Rev. B 33, 3549 (1986)
Jaeger, H.M., Haviland, D.B., Goldman, A.M., Orr, B.G.: Phys. Rev. B 34, 4920 (1986)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boussouf, N., Mosbah, MF., Amira, A. et al. Effect of ZrO2 Addition on Microstructure, Transport and Magnetic Properties of Bi2Sr2CaCu2O8+δ System. J Supercond Nov Magn 26, 1105–1109 (2013). https://doi.org/10.1007/s10948-012-1977-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-012-1977-3