Abstract
The polycrystalline Bi1.8Pb0.4Sr2.0Ca1.1Cu2.1 MxO y , with M = Zr (x = 0.0, 0.02, 0.04), were synthesized by solid-state reaction method and studied by X-ray diffraction analysis (XRD), scanning electron microscopy equipped with energy dispersive of X-ray analysis (SEM/EDX) and resistivity versus temperature measurements. The influence of the Zr addition on the Tc and microstructure properties of the superconducting compounds has been studied. SEM observations show whiskers grains randomly distributed and microstructural change due to the addition of Zr. The ZrO2 was incorporated into the crystalline structure of BSCCO system in all samples. The crystallographic structure remains in a tetragonal form where a= b≠c. Generally, all samples exhibit semiconductor behaviour above \(T_{\mathrm {c}}^{\text {onset}}\). The onset critical temperature \(T_{\mathrm {c}}^{\text {onset}}\) increases up to 86 with x = 0.02. There is an enhancement in the critical temperature for doped samples as compared with pure Bi1.8Pb0.4Sr2.0Ca1.1Cu2.1O y .Changes in superconducting properties of ZrO2 nanoparticle added Bi-2212 system were discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Since the discovery of BSCCO high-temperature superconductors, there has been tremendous effort to improve formation of the (2212) phase with different parameters processing of composite materials. Properly distributed in the superconductor matrix fine inclusions of impurities phases may act as strong pinning centres, favour the development of textured microstructure with good intergrain superconducting connections and improve mechanical properties of the material.
The dopant materials like Ag, Ti, Cr, Nb, Sb and Pb have been reported by different authors in the Bi-based superconductors [1–7]. Dopant may play an important role on the properties of high-temperature superconducting materials to reach higher Tc and critical current density. The effect of nano sized MgO powder addition to Bi (2212) in the weight ratio 95:5 will change the Tc and also the Jc. It was reported that the Tc of the Bi (2212) single crystal was 93 K but it decreases to 92 K after doping. In addition, several methods had been reported in the literature such as adding impurity phases to the superconductor like Y2Ba2CuO2, columnar defects generated by ion irradiation, point defects generated by oxygen deficiency, or chemical substitution [8].
Addition of perovskites SrAO3 (A = Zr, Sr etc.) with Bi-2212 matrix shows the partial incorporation of the particles into Bi-2212 grains and partially agglomerated, forming the thin closed areas between the Bi-2212 lamellas and due to this the Jc increases and Tc decreases. The dopants’ effect on enhancement and stabilization of high-Tc phase has been reported earlier [9, 10]. Previous investigations [11, 12] have showed that LiF addition to Bi2Sr2CaCu2 O 8 + δ and Bi1.8Pb0.4Sr1.8Ba0.2Ca2Cu3 O x superconducting system leads to a significant grow of the critical current density by increasing of the Bi-2212, Bi2223 superconducting phase content accompanied by a slightly increase of the critical temperature.The studies on Pr addition showed an increase in room temperature resistivity and increase in the critical temperature (Tconset) with increasing amount of addition [13].
The present work reports the influence of the Zr addition on the superconducting phase formation and the transport properties of the BPSCCO system, synthesized by solid-state reaction. Based on previous results, we are assuming that Zr ion can substitute Ca ions in the Bi (Pb) 2212 phase. The dopant can influence the kinetics and mechanism of HSTC phase formation, thus changing the final microstructure of the superconductor. We aim to study the effect of Zr on the Bi-based superconductors grown by solid-state reaction.
2 Experimental Procedure
The Zr-doped BPSCCO ceramics were prepared by the conventional ceramic method. Predetermined amounts of high purity starting chemicals (Bi2 O 3; SrCO3, CaCO3, CuO, PbO) were used for the preparation of the ceramics of fixed nominal composition of Bi1.8Pb0.4Sr2Zr x Ca1.1Cu2.1O y . These powders were well mixed and ground by using an agate mortar and pestle and were further calcined at 800∘C for 25 h in a furnace. Calcined powders were ground again to form a fine powder. Pellets 13 mm in diameter and 1 mm in thicknesses were pressed by uniaxial compaction in a die at 5 ton/cm2. These pellets were sintered at 840∘C for 60 h in air and then furnace cooled to room temperature. The obtained Bi (Pb)-2212 powders were mixed with ZrO2 particles. The additional amount of ZrO2 in this case varying from (x = 0, 2 and 4 %) of the total mass of the sample. The mixed powders were pressed into pellets under 5 ton/cm2 then sintered at 825∘C for 75 h. For comparison, sample with Mg substitution was also prepared by the same processing route with mixing, appropriate amounts of high purity powders, Bi2 O 3, SrCO3, CaCO3, CuO, PbO and ZrO2, in the correct stoichiometric proportions Bi1.8Pb0.4Sr2Zr x Ca1.1Cu2.1O y and ground in an agate mortar.
Phase identification was carried out with an X-ray diffractometer (DRX). The cell parameters were calculated From XRD patterns using Dicvol 06. Resistance-temperature data were obtained by using four point probe AC method.
3 Results
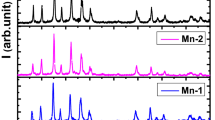
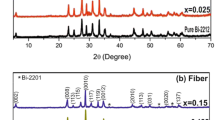
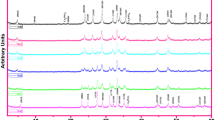
The XRD patterns of the samples are shown in Fig. 1. The XRD studies show that Bi-2212 phase exist in most of the samples [14]. The result indicates that on doping the structure of the BSCCO does not change and the major peaks still corresponds to the 2212. Besides these prominent peaks, some other peaks of low intensity at 21∘, 26∘ and 30∘ angles are also observed. Indicating the presence of the Bi2201 parasitic phase [15] and other oxides identified as CuO and Ca2CuO3 [16–18], SrZrO3 particles are both aggregated between the lamellas in the closed elongated areas with a thickness of 1–3 μm and a length of several μm, and imbedded in Bi2212 matrix as separate grains. The increase in the intensities of the peaks of doped samples may testify to the enhanced grain growth and orientation of grains. The secondary phase Ca2PbO4 was detected at 2 𝜃 = 17.85∘, 45.1∘ [19]. The overlapping secondary phase reduces at that point. The major secondary phases involved directly in sequential reaction are Ca2PbO4 and CuO.
No peaks of ZrO2 were detected in the diffraction patterns for sample doped with 0.02 meaning that this oxide was incorporated into the crystalline structure in the BSCCO system. The intensity of peaks (008), (113), (0010), (115) and (117) were observed to increase in sample x = 0.02 as compared to that of Zr-free sample.
Figure 2 shows the SEM image of the surface morphology of the samples (x = 0, 1, 2 and 4 %), respectively. The Zr-free Bi1.8Pb0.4Sr2.0Ca1.1Cu2.1O y sample consists of flake type grains and some needle type grains. The grains are distributed randomly [20, 21] or we can say that the grains in the specimen are oriented anisotropically and poorly connected. The surface of sample doped with 1 % is smoother and denser and the grain is longer than the other doped samples. For the 2 and 4 % doped Zr shown in Fig. 2 with a slight different microstructure, which is probably due to composition shift, the grain size of the 2 % sample decreases, which shows further decrease in grain size with 4 % addition of Zr. We have seen more white space in 4 % doped sample, which indicates the presence of ZrO2.
The EDX plot reveals no extra peaks and reflects the presence of all the constituents. All the samples show the exact match for standard peak position for Bi, Sr, Ca, Cu, Pb, Zr and oxygen (O). This reveals that the elemental composition of all the samples do not contain any foreign element [22]. It is observed from the graph that with the addition of Zr in BPbSCCO, the density of Sr, Cu increases (Fig. 3). The change in ion amounts due to addition is shown Tables 1, 2 and 3.
The electrical resistivity versus temperature curve for the entire sample, from 300 K (room temperature) down to 70 K for different compositions (x = 0, 2 and 4 %) are shown in the respective Fig. 4. It is found that all the samples show semiconductor behaviour above the Tc-onset value. Tc-onset transition temperature of three different samples is found to be about 81, 87 and 82 K, respectively. The phase transition of BSCCO from semiconductor state to superconducting state happens at temperature 70 and 76 K for BSCCO doped with 2 and 4 %, respectively. It is observed that the zero resistivity transition temperature for sample 0.02 is higher than that of free sample. The increase of Tc may be related to the optimization of the hole concentration and possible change of the lattice vibration of Bi(Pb)-Sr-Ca-Cu-O. It is also possible that resistive nature of the grain boundaries is modified by accumulation of Zr at the grain boundaries. The transition width of the doped sample is increase for sample doped with 4 %. The broadening of the transition width shows that free sample has lower percentage of Bi2212 phase compared to that of doped samples. The large increase of the grain size observed for the highest content of Zr is then obtained with a degradation of the grain boundaries. Thus, high content of Zr can lead to an increase of the weak links [23]. This behaviour can be also explained, when high rate is used, by the substitution of the spinless Zr+4 ion in the Cu+2 site. The perturbation of the local magnetic ordering can destroy the coherence of the phase ϕ of the order parameter ψ [24, 25], and results in pair breaking effect [26, 27].
The reduction of the broadening in ρ-T curve may be due to the modifying effect of Zirconium diffusion on the grain boundaries.
4 Conclusion
Samples with Zirconium addition show that the Zr addition increases the reactivity of the precursor and favours the formation of 2212. The results presented that the adding of zirconium enhances the superconducting properties of doped samples and improve the Tc.
From the SEM study of ZrO2-added BSCCO, the presence of rounded shaped Zr having small grain size is observed in needle-shaped BSCCO. The decrement in grain size was observed. EDX plot reveals no extra peaks related to elements other than the constituents. From resistivity vs. temperature measurement, the superconducting transition temperature Tc of the parent BSCCO is found to be down of 70 K. With increase in concentration of Zr in BSCCO, the transition temperature decreases to 70 and 76 K for 2 and 4 %, respectively.
References
Deis, T. A., Lelovic, M., Eror, N. G., Balanchandran, U. Appl. Supercond. 6(6), 279–284 (1998)
Galvan, D. H., Durán, A., Castillón, F. F., Adem, E., Escudero, R., Ferrer, D., Torres, A., José-Yacamán, M.: J. Supercond. Nov. Magn. 21, 271–277 (2008)
Hamid, N. A., Abd- Shukor, R.: J. Mater. Sci. 35, 2325–2329 (2000)
Weli, Kong, Abd-Shukor, R.: J. Electron. Mater. 36(12) (2007)
Azhan, H., Azman, K., Yusainee, S. Y. S.: Solid State Science and Technology 17(1), 215–221 (2009)
Biju, A., Sarun, P. M., Aloysius, R. P., Syamaprasad, U.: Supercond. Sci. Technol. 19, 1023 (2006)
Sotelo, A., Mora, M., Madre, M. A., Amave da, H., Diez, J. C., Angurel, L. A., Mayoral, M. C.: Bol. Soc. Esp. Ceram. 45(3), 228–232 (2006)
dos Santos, D. I., Rodrigues, JrD., Rubo, E. A. A., Cursino, E.: Revista Brasileira de Aplicações de Vácuo 23(1), 24– 26 (2004)
Maqsood, A., Khaliq, M., Maqsood, M.: J. Mat. Sci. 27, 5330 (1992)
Khan, M. N., Kayani, A. N., Ul.Haq, A.: J. Mat. Sci. 33, 2365 (1998)
Matsubara, I., Tanigawa, H., Ogura, T., Yamashita, H., Kinoshita, M., Kawai, T.: Phys. C 167, 503 (1990)
Velter-Stefanescu, M., Duliua, O. G., Mihalache, V.: J. Optoelectron. Adv. Mater. 7(3), 1557–1561 (2005)
Ozturk, O., Asikuzun, E., Erdem, M., Yildirim, G., Yildiz, O., Terzioglu, C.: J Mater Sci: Mater Electron 23, 511–519 (2012)
Suazlina, M. A., Yusainee, S. Y. S., Azhan, H., Abd-Shukor, R., Mustaqimd, R. M.: J. Teknologi Sci. Eng. 69(2), 49–52 (2014). eISSN 2180–3722. www.jurnalteknologi.utm.my.
Zhao, B., Song, W. H., Wu, X. C., Du, J. J., Sun, Y. P., Wen, H. H., Zhao, Z. X.: Phys. C 361, 383–391 (2001)
Arshad, M., Qureshi, A. H., Masud, K., Qazi, N. K.: J. Therm. Anal. Calorim. 89(2), 595–600 (2007)
Guilmeau, E., Andrzejewski, B., Noudem, J. G.: Phys. C 387, 382–390 (2003)
Nagao, M., Sato, M., Maeda, H., Kim, S., Yamashita, T.: Phys. C 377, 260–266 (2002)
Zhao, B., Song, W., Wan, X., Sun, Y., Du, J.: Phys. C 337, 145–149 (2000)
Kazin, P. E., Jansen, M., Larrea c, A., de la Fuente, G. F., Tretyakov, Y.D.: Phys. C 253, 391–400 (1995)
Vinu, S, Sarun, P M, Biju, A, Shabna, R, Guruswamy, P, Syamaprasad, U.: Supercond. Sci. Technol. 21, 045001 (2008)
Boussouf, N., Mosbah, M.-F., Amira, A., Varilci, A., Altintas, S. P., Guerioune, M.: J. Supercond. Nov. Magn. 26, 1105–1109 (2013)
Nkum, R. K., Datars, W.: Supercond. Sci. Technol. 8, 822 (1995)
Dynes, R. C., White, A. E., Graybeal, J. M., Garno, J. P.: Phys. Rev. Lett. 57, 2195 (1986)
Graybeal, J. M., Beasley, M. A.: Phys. Rev. B 29, 4167 (1984)
White, A. E., Dynes, R. C., Garno, J. P.: Phys. Rev. B 33, 3549 (1986)
Jaeger, H. M., Haviland, D. B., Goldman, A. M., Orr, B. G.: Phys. Rev. B 34, 4920 (1986)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boussouf, N., Mosbah, M.F., Kalkoul, N. et al. Effect of Zr Addition on Bi1.8Pb0.4Sr2.0Ca1.1Cu2.1O y Superconductor. J Supercond Nov Magn 30, 365–370 (2017). https://doi.org/10.1007/s10948-016-3728-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-016-3728-3