Abstract
This study examines the significant changes in the structural and superconducting properties of cerium (Ce) doped Bi-2212 superconductors via X-ray diffraction analysis (XRD), scanning electron microscopy (SEM), electron dispersive X-ray (EDX), electrical resistance and transport critical current density (J c ) measurements. Ce concentration is varied from x=0.0 until 0.1 in a general stoichiometry of Bi1.8Sr2.0Ce x Ca1.1Cu2.1O y . Zero resistivity transition temperatures (\(T_{c}^{\mathrm{offset}}\)) of the samples produced by the conventional solid-state reaction method are deduced from the dc resistivity measurements. Furthermore, the phase fractions and lattice parameters are determined from XRD measurements when the microstructure, surface morphology and element composition analyses of the samples are investigated by SEM and EDX measurements, respectively. The results show that \(T_{c}^{\mathrm{offset}}\) and J c at self-field of the samples reduced gradually with the increase in the Ce addition. Maximum \(T_{c}^{\mathrm{offset}}\) of 79.7 K and J c of 356.8 A⋅cm−2 at 77 K are obtained for pure sample as against 44.6 K and 18.7 A⋅cm−2, respectively, for the sample doped with 0.1 wt.% Ce. According to the refinement of cell parameters done by considering the structural modulation, the Ce doping is confirmed by both an increase of the lattice parameter a and a decrease of the cell parameter c of the samples in comparison with that of the pure sample. As for SEM measurements, it is found that not only do the surface morphology and grain connectivity degrade but the grain size of the samples also decreases with the increase of the Ce addition. Moreover, EDX images indicate that the elements used for the preparation of samples distribute homogeneously and the Ce atoms enter into the crystal structure by replacing Cu atom. In addition, the variation of ΔT c (\(T_{c}^{\mathrm{onset}} -T_{c}^{\mathrm{offset}}\)) is investigated for the presence of impurities and weak links between superconducting grains of the samples. The possible reasons for the degradation in microstructural and superconducting properties are also interpreted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the discovery of Bi–Sr–Ca–Cu–O (BSCCO) high-temperature superconductors in 1998 [1], the interest in both the fundamental research and applications in technology and industry of these materials has been increased day by day owing to their remarkable high transition temperature, smaller power losses, high current and magnetic field carrying capacity, optical and electronic properties [2–4]. The BSCCO system obtaining a layered structure has three different phases with regard to its chemical compositions, the Bi-2201 phase (n=1, T c ≈20 K), the Bi-2212 phase (n=2, T c ≈85 K) and the (Bi, Pb)-2223 phase (n=3, T c ≈110 K) [5]. Among the Bi-based superconductors, the double layered cuprate (Bi-2212) is superior to the others because of the fact that its oxygen stoichiometry is relatively invariant with regard to cationic addition when the samples are produced in an identical thermal environment [6–8]. Furthermore, the Bi-2212 superconductors have lesser weak link problems compared to Bi-2223 superconductors and are suitable for the preparation of long wires and tapes with high critical current densities (J c ). However, as received the applied magnetic fields and high temperatures limit their applications due to the significant reduction in condensation energy density, strong anisotropic properties, extremely short coherence length (ξ), and large penetration depth (λ) [9, 10]. Likewise, according to the many researches [11–13], the Bi-2212 superconductors have relatively poor mechanical properties, limiting the use of these materials in most of practical applications [14]. Therefore, several methods have been studied to improve their mechanical, superconducting and flux pinning properties to make them suitable for high-temperature and magnetic field applications for years [15, 16]. Chemical doping in the superconductor materials is one of the most preferred methods [17–20] because the changes obtained by the chemical doping can be used to investigate the mechanism of high-temperature (high-T c ) superconductivity [21–24]. The critical current density and the critical temperature might be increased by the doping. The improvement of the critical current density is attributed to the enhancement of trapped flux in the superconductor material [25, 26] and single-domain structure [27, 28]; similarly, the increase in the critical temperature is associated with the enhancement of average Cu valency. Thus, the increased density of mobile holes in the CuO2 planes [29] improves the superconductivity of the system [30]. However, sometimes the chemical doping method causes to the decrement in the number of charge carriers (either holes or electrons) in the materials and so the superconducting properties are regressed [31, 32].
The aim of the present work is to examine the effect of Ce addition on the structural and superconducting properties of Bi-2212 ceramics prepared by conventional solid-state reaction with the aid of the dc electrical resistivity, critical current density, XRD, SEM and EDX measurements. Dc electrical resistivity and critical current density for electrical and superconducting properties, XRD measurements for the phase composition and lattice parameters, SEM and EDX images for microstructure examination and element composition analyses of the samples produced are performed, respectively. The results illustrate that the Ce doping in the Bi-2212 ceramics leads to not only reduce the charge carrier numbers and grain sizes but suppress the surface morphology and grain connectivity, as well.
2 Experimental Details
In this study, samples with the nominal stoichiometry of Bi1.8Sr2.0Ce x Ca1.1Cu2.1O y (0≤x≤0.1) are elaborated in air by conventional solid-state reaction [33] using high purity oxides and carbonates (Alfa Aesar Co., Ltd. 99.9% purity). The accurately weighted ingredients are grounded homogeneously by means of a grinding machine for 12 hours and calcined in a tube furnace (Protherm-Model PTF12/75/200) at 800 °C for 48 hours with a heating rate of 5 °C /min. The powders calcined are pelletized into rectangular bars with dimensions of 10 mm ×4 mm×2 mm under a pressure of 330 MPa. The sintering process of the pellets is performed at 840 °C for 48 hours in the furnace. The heating and cooling rates of the furnace are adjusted to be 3 °C/min and 5 °C/min, respectively. The superconductor samples prepared with different Ce stoichiometry such as x=0, 0.001, 0.003, 0.005, 0.01, 0.03, 0.05 and 0.1 will be herein after denoted as Ce0, Ce1, Ce2, Ce3, Ce4, Ce5, Ce6, Ce7 and Ce8, respectively.
The dc resistivity measurements are carried out on the surface of the samples by the four-probe method. Both voltage and current contacts are made with silver paint to minimize the contact resistance. The temperature (5–110 K) dependence of resistance is measured by running 5 mA dc current through the sample in the cryostat. A Keithley 220 programmable current source and a Keithley 2182A nano-voltmeter are used for the resistance measurements. Additionally, the transport critical currents of the samples in self-field are measured by home made system at 77 K in zero field using four-probe method. A programmable temperature controller (Lakeshore 340) is used for the accurate monitoring of the temperature with a stability and accuracy of ±0.01 K. The current applied parallel to the direction of the pressed surface is ramped at a constant rate (0.02 A/s) for the entire sample. The critical current (I c ) values of the samples are defined with the criterion of 1 µV/cm. The J c values of the samples produced are calculated from the I c and the total cross-sectional area (width of 4 mm× thickness of 2 mm). All the data obtained are recorded using the Labview computer software. Furthermore, the structural and phase analyses of the samples studied are characterized by XRD measurements by using a Rigaku multiflex diffractometer XRD with Cu K α radiation (λ=1.5418 Å) in the range 2θ=3−60° at a scan speed of 3°/min and a step increment of 0.02∘ at room temperature. Phase purity and lattice parameters are estimated from the XRD patterns. The accuracy in determining the lattice parameters (a and c) is ±0.0001 Å. Average sizes of the crystal of the samples prepared are also found by the Scherrer–Warren approach [34, 35]. Moreover, the surface morphology, grain connectivity and grain size of the samples are examined with the aid of a Jeol scanning electron microscope (SEM) JEOL 6390-LV, operated at 20 kV, with a resolution power of 3 nm. The elemental compositions (in the percent) and distributions (homogeneously or not) of the samples are analyzed by means of an Oxford X-ray micro-probe analysis connected to SEM. The bulk densities of the samples are also determined from their mass and geometrical dimensions to obtain the information about the structure (porosity) of the samples produced in this work.
3 Results and Discussion
3.1 Electrical Resistance Measurements
The temperature dependence of the electrical resistance (R–T) for Bi1.8Sr2.0Ce x Ca1.1Cu2.1O y with x=0.0, 0.001, 0.003, 0.005, 0.01, 0.03, 0.05 and 0.1 is depicted in Fig. 1a. It is observed that all the samples show metallic behavior above the critical temperature. The normal-state resistance (Table 1) is found to systematically enhance with the increase in the Ce addition to a maximum (4.08 Ω) for x=0.1 as against 1.21 Ω for the pure sample. When Ce4+ ions are doped in the system, each doping of Ce4+ fills one hole in the crystal. Hence, the hole concentration in the Cu–O plane of the system decreases and normal-state conductivity of the system increases. Besides, the onset critical (\(T_{c}^{\mathrm{onset}}\)) and offset critical (\(T_{c}^{\mathrm{offset}}\)) temperatures deduced from the normalized resistance graphs (Fig. 1b) are given in Fig. 2. As can be seen from the figure, the offset critical temperatures decrease gradually whereas the onset temperatures exhibit a wavy change with the increase of Ce-content. \(T_{c}^{\mathrm{offset}}\) value is found to be about 79.7 K for the Ce0 and 44.6 K for the Ce7 sample. On the other hand, the maximum \(T_{c}^{\mathrm{onset}}\) is obtained for the Ce6 sample (94.4 K) and thereafter it decreases to 91.6 K for the Ce7 sample. The variation of ΔT c (\(T_{c}^{\mathrm{onset}} - T_{c}^{\mathrm{offset}}\)) is also shown in Fig. 3, indicating that ΔT c increases with increase in Ce-content up to x=0.1 sample. When the ΔT c value is observed to be about 6.5 K for the undoped sample, the ΔT c of Ce7 is obtained to be about 47.1 K (Table 1). Additionally, the broadening of the resistive transition increases as the Ce-content enhances (Fig. 1). This phenomenon indicates the presence of impurities and weak links between the superconducting grains [36], supporting the results of the SEM measurements. Consequently, the \(T_{c}^{\mathrm{offset}}\) values responsible for superconductivity are observed to considerably decrease with the increase of the Ce addition.
3.2 Hole-Carrier Concentration Calculation
The hole-carrier concentrations per Cu ion, P, are calculated by means of the following relation [37]:
where \(T_{c}^{\max}\) is received as 85 K for Bi-2212 phase [38] and 110 K for (Bi, Pb)-2223 phase [39] and \(T_{c}^{\mathrm{offset}}\) values are taken form Table 1. The P values are obtained to decrease from 0.1572 to 0.0841 and are tabulated in Table 1. The variations of hole-carrier concentrations as a function of Ce-content and superconducting transition temperature (\(T_{c}^{\mathrm{offset}}\)) are also depicted in Fig. 4. It is visible that the left panel of the figure illustrates the decrement of the hole-carrier concentration with increasing Ce addition and the right panel of the figure shows the parabolic relationship between the \(T_{c}^{\mathrm{offset}}\) and hole-carrier concentrations. According to these results, the Ce addition in the Bi-2212 system reduces remarkably the hole-carrier concentration, leading to the decrease in the superconducting properties of the system. Moreover, the reducing trend in the bulk density of the Ce-doped samples is observed because of the increase in the porosity of the samples prepared in this work (Table 1).
3.3 Transport Critical Current Density
Figure 5 reveals the role of the Ce-content on the self-field critical current density (J c ) of the Bi-2212 ceramics produced. The self-field J c is found to decrease significantly for Ce-doped samples (Fig. 5a). The undoped sample indicates a J c of 356.8 A/cm2 that is the maximum current density in the samples prepared while the minimum J c obtained is 18.7 A/cm2 for x=0.1 sample at 77 K (in liquid nitrogen), indicating that the J c values of the sample decrease dramatically as the Ce-content increases (Table 2). This suppression of J c is attributed to the increase of the weak links between the superconducting grains, porosity, secondary phases, grain boundaries resistance and de-orientation of Bi-2212 grains with the increase in the Ce addition [40, 41], supporting the results of the structural investigations. Moreover, Fig. 5b presents the parabolic variation between the superconducting transition temperatures and critical current densities of the samples. Although both the J c and T c values decrease with the increase in Ce addition, a sharper decline is observed in J c values compared to the decrement of T c values. In other words, the critical current density is found to be more sensitive to the change of Ce-content.
3.4 SEM Analyses
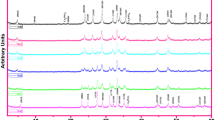
The surfaces of the samples prepared are imaged by Scanning Electron Microscopy (SEM) method. Figure 6a–h depicts the SEM pictures (taken in the secondary electron image mode) of the fractured surface of the Ce0, Ce1, Ce2, Ce3, Ce4, Ce5, Ce6 and Ce7 samples, respectively. It is clearly seen that surface morphology changes with addition of the Ce atoms in the Bi-2212 system. The grain morphology of the pure sample illustrates the clear and flaky grains with layered growth [42]. Moreover, the texturing and layered grain growth of the pure sample are found to be better compared to the doped samples. On the other hand, the grain size and texturing of the doped samples are decreased compared to the pure sample, indicating that these results are in good agreement with the Lotgering index data obtained. Using the image processing, the average grain sizes are also obtained to decrease from 540 nm to 350 nm with the Ce doping. Based on the SEM results, the surface morphology, grain connectivity and average crystallite size degrade with the increase of the Ce addition.
3.5 EDX Analyses
The quantitative values of the atomic compositions present in the Bi-2212 grains of samples are investigated by the electron dispersive X-ray (EDX). Figure 7 presents the EDX analysis for Bi1.8Sr2.0Ca1.1Cu2.1O y and Bi1.8Sr2.0Ce0.1Ca1.1Cu2.1O y . As seen from the figure, no difference appears between the two samples produced except for Ce peak, confirming that the Ce atoms are successfully introduced to the microstructure of the Bi-2212 phase. Moreover, Bi, Sr, Ca, Cu, O, and Ce element composition mappings taken on the surface of all the samples prepared in this work are displayed in Fig. 8a–f. It is clear that the elements used for the preparation of samples are observed to distribute homogeneously. However, the Cu compositions compared to other element compositions decrease rapidly with the increase in the Ce addition. According to the EDX results, the Ce atoms might enter into the crystal structure by replacing Cu atoms [43].
3.6 XRD Analyses
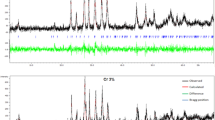
The XRD patterns of the samples produced are depicted in Fig. 9. The corresponding (h k l) Miller indices belonging to Bi-2212 main lines are shown in the diagrams. As can be seen from the figure, both pure and Ce-doped samples contain the Bi-2212 phase only and all the samples studied exhibit the polycrystalline superconducting phase with less intensity of diffraction lines with increasing the Ce addition. Moreover, no secondary phase containing Ce or any other cation is observed for all the samples, indicating that Ce atoms are incorporated into the crystal structure of Bi-2212 superconductor [44–46]. The peak intensity of the doped samples reduces regularly and other peaks such as 315, 006 and 30 10 disappear completely as Ce addition increases, revealing that why the superconducting properties degrade with the Ce doping in the system. Moreover, the lattice parameters a and c are calculated using the least square method through d values and (h k l) planes for tetragonal unit cell structure. The a and c parameters obtained are listed in Table 2. It is visible that a systematic expansion in the a-axis length is observed [47, 48] while a regular contraction in the c-axis length is obtained with the increase in the Ce addition [49, 50]. It is well known that the lattice parameter a is controlled by the length of in-plane Cu–O bond [19]. The length may be expanded or contracted with the change of the electrons into antibonding orbital. In our system this bond length is expanded because of the partial replacement of Cu2+ ions by Ce4+ ions, leading to an increase in the lattice parameter a, and a decrease in the lattice parameter c. The variation of the lattice parameters as a function of the Ce-content is also depicted in Fig. 10. As seen from the figure, there is a considerable increase in the a parameter and decrease in the c parameter. Based on these results, it might be interpreted that the Bi-2212 phase decreases while the Bi-2201 phase starts to increase with the increase of the Ce addition; however, Fig. 9 confirms that the Bi-2212 phase is dominant for all the samples studied. These findings are also ascertained by both the resistivity and SEM measurements. Furthermore, Lotgering index (F) is investigated to quantify the degree of c-axis orientation in polycrystalline ceramics produced in this work [51]. The Lotgering indices computed by means of the XRD patterns [52] are tabulated in Table 2. It is found that the texturing of Bi-2212 grains decreases monotonically with the increase of the Ce-content, supporting the SEM investigations.
Additionally, the broadening nature of the XRD peaks shows that the particle sizes determined from the Scherrer–Warren equation are within the nanometer scale [53–56]. According to the equation, in broadening region the average size of a crystal is defined as;
where d is the thickness of the crystal, λ is the wavelength, B is the fullwidth half maximum (FWHM) of the Bragg peak corrected using the corresponding peak in micron-sized powder and θ B is the Bragg angle. Moreover,
where B s is the halfwidth of the standard material in radians. The grain size of the pure sample is calculated to be 537 nm whereas that of Ce7 sample is obtained to be about 356 nm (minimum value). Based on the calculations, the grain size of the samples studied decreases dramatically with increasing the Ce doping (Fig. 11).
4 Conclusion
In this study, the effect of Ce doping on the structural and superconducting properties of Bi1.8Sr2.0Ce x Ca1.1Cu2.1O y superconductors prepared by the conventional solid-state reaction with x=0, 0.001, 0.003, 0.005, 0.01, 0.03, 0.05 and 0.1 is analyzed by means of the XRD, SEM, EDX, R–T and I–V measurements. It is found that the zero resistivity transition temperatures and transport critical current densities decrease from 79.7 K to 44.6 K and from 356.8 A/cm2 to 18.7 A/cm2, respectively, as Ce-content increases. Further, XRD investigations show that the cell parameter a increases while the c parameter decreases with increasing the Ce addition. SEM results reveal that the surface morphology and grain connectivity degrade and the grain sizes of the samples decrease from 537 nm to 356 nm with the increase of the Ce addition. As for the EDX measurements, it is seen that not only do the Cu compositions in the samples compared to other element compositions gradually decrease with the increasing the Ce doping but the elements used for the preparation of samples distribute homogeneously, as well. According to these results, the Ce atoms enter into the crystal structure by replacing Cu atoms, leading to the degradation of the structural and superconducting properties of the samples studied in this work. Besides, the bulk densities determined from their mass and geometrical dimensions reduce slightly at a constant rate with the increase of x because of the porosity structure formation, confirming T c and J c values of the samples studied depend strongly on the microstructure. Based on the Lotgering index, the degree of c-axis orientation in polycrystalline ceramics produced is also found to decrease with the Ce doping.
References
Maeda, H., Tanaka, Y., Fukutomi, M., Asano, T.: Jpn. J. Appl. Phys. 27, L209 (1988)
Runde, M.: IEEE Trans. Appl. Supercond. 5, 813 (1995)
Godeke, A., Cheng, D., Dietderich, D.R., English, C.D., Felice, H., Hannaford, C.R., Prestemon, S.O., Sabbi, G., Scanlan, R.M., Hikichi, Y., Nishioka, J., Hasegawa, T.: IEEE Trans. Appl. Supercond. 18, 516 (2008)
Miao, H., Meinesz, M., Czabai, B., Parrell, J., Hong, S.: AIP Conf. Proc. 986, 423 (2008)
Mousavi Ghahfarokhi, S.E., Zargar Shoushtari, M.: Physica B 405, 4643 (2010)
Awana, V.P.S., Agarwal, S.K., Ray, R., Gupta, S., Narlikar, A.V.: Physica C 43, 191 (1992)
Tarason, J.M., Barboux, P., Hull, G.W., Ramesh, R., Greene, L.H., Giroud, M., Hegde, M.S., Mckinnon, W.R.: Phys. Rev. B 39, 4316 (1989)
Koyama, K., Kanno, S., Noguchi, S.: Jpn. J. Appl. Phys. 29, L53 (1990)
Vinu, S., Sarun, P.M., Shabna, R., Aswathy, P.M., Anooja, J.B., Syamaprasad, U.: Physica B 405, 4355 (2010)
Vinu, S., Sarun, P.M., Shabna, R., Syamaprasad, U.: J. Alloys Compd. 487, 1 (2009)
Yilmazlar, M., Cetinkara, H.A., Nursoy, M., Ozturk, O., Terzioglu, C.: Physica C 442, 101 (2006)
Leenders, A., Ullrich, M., Freyhardt, H.C.: Physica C 279, 173 (1997)
Yilmazlar, M., Ozturk, O., Gorur, O., Belenli, I., Terzioglu, C.: Supercond. Sci. Technol. 20, 365 (2007)
Sedky, A.: Physica C 468, 1041 (2008)
Ghazanfari, N., Kilic, A., Gencer, A., Ozkana, H.: Solid State Commun. 144, 210 (2007)
Makise, T., Uchida, S., Horii, S., Shimoyama, J., Kishio, K.: Physica C 460–462, 772 (2007)
Ozturk, O., Akdogan, M., Aydin, H., Yilmazlar, M., Terzioglu, C., Belenli, I.: Physica B 399, 94 (2007)
Terzioglu, C., Aydin, H., Ozturk, O., Bekiroglu, E., Belenli, I.: Physica B 403, 3354 (2008)
Sarun, P.M., Vinu, S., Shabna, R., Biju, A., Syamaprasad, U.: Mater. Res. Bull. 44, 1017 (2009)
Sidorenko, A., Scheidt, E.W., Haider, F., Klemm, M., Horn, S., Konopko, L., Tidecks, R.: Physica B 321, 298 (2002)
Eisaki, H., Kaneko, N., Feng, D., Feng, V., Damascelli, A., Mang, P.K., et al.: Phys. Rev. B 69, 064512 (2004)
Kapitulnik, A., Fang, A., Howald, C., Greven, M.: J. Phys. Chem. Solids 67, 344 (2006)
Tsuei, C.C., Kirtley, J.R., Hammerl, G., Mannhart, J., Raffy, H., Li, Z.Z.: J. Phys. Chem. Solids 67, 64 (2006)
Fujita, K., Noda, T., Kojima, K.M., Eisaki, H., Uchida, S.: Phys. Rev. Lett. 95(1–4), 097006 (2005)
Ren, Y., Weinstein, R., Liu, J., Sawh, R.P., Foster, C.: Physica C 251, 15 (1995)
Ikuta, H., Mase, A., Yanagi, Y., Yoahikawa, M., Itoh, Y., Oka, T., Mizutani, U.: Supercond. Sci. Technol. 11, 1345 (1998)
Xu, X.Q., Cai, Y.Q., Yang, C.X., Yao, X., Xu, S., Kortyka, A., Puzniak, R.: Supercond. Sci. Technol. 22, 015001 (2009)
Xu, Y., Izumi, M., Tsuzuki, K., Zhang, Y.F., Xu, C.X., Murakami, M., Sakai, N., Hirabayashi, I.: Supercond. Sci. Technol. 22, 095009 (2009)
Chevalier, B., Lepine, B., Lalerzin, A., Darriet, J., Eournau, J., Tarascon, J.M.: Mater. Sci. Eng. B 2, 277 (1989)
Jayaram, B., Lanchester, P.C., Weller, M.T.: Physica C 160, 17 (1989)
Guo, Y.C., Horvat, J., Liu, H.K., Dou, S.X.: Physica C 300, 38 (1998)
Yildirim, G., Zalaoglu, Y., Akdogan, M., Altintas, S.P., Varilci, A., Terzioglu, C.: J. Supercond. Nov. Magn. doi:10.1007/s10948-011-1174-9
Yildirim, G., Akdogan, M., Altintas, S.P., Erdem, M., Terzioglu, C., Varilci, A.: Physica B 406, 2011 (1853)
Cullity, B.D.: Elemt of X-ray Diffraction, 3rd edn. Addition-Wesley, Reading (2001)
Yucel, E., Terzioglu, C., Varilci, A., Belenli, I.: J. Mater. Sci. Mater El. (2011). doi:10.1007/s10854-010-0274-9
Ianculescu, A., Gartner, M., Despax, B., Bley, V., Leby, Th., Gavrila, R., Modreanu, M.: Appl. Surf. Sci. 253, 344 (1996)
Azzouz, B.F., Mchirgui, A., Yangui, B., Boulesteix, C., Salem, B.M.: Physica C 356, 83 (2001)
Kucukomeroglu, T., Bacaksiz, E., Terzioglu, C., Varilci, A.: Thin Solid Films 516, 2913 (2008)
Persland, M.R., Tallon, J.L., Buckley, R.G., Liu, R.S., Floer, N.E.: Physica C 176, 95 (1991)
Sarun, P.M., Vinu, S., Shabna, R., Biju, A., Syamaprasad, U.: Mater. Lett. 62, 2725 (2008)
Abou-Aly, A.I., Mahmoud, S.A., Awad, R., Barakat, M.M.E.: J. Supercond. Nov. Magn. 23, 1575 (2010)
Moodera, J.S., Meservey, R., Tkaczyk, J.E., Hao, C.X., Gibson, G.A., Tedrow, P.M.: Phys. Rev. B 37, 619 (1988)
Biju, A., Syamaprasad, U., Rao, A., Xu, J.G., Sivakumar, K.M., Kuo, Y.K.: Physica C 466, 69 (2007)
Vinu, S., Sarun, P.M., Biju, A., Shabna, R., Guruswamy, P., Syamaprasad, U.: Supercond. Sci. Technol. 21, 045001 (2008)
Vinu, S., Sarun, P.M., Shabna, R., Biju, A., Syamaprasad, U.: Mater. Lett. 62, 4421 (2008)
Shabna, R., Sarun, P.M., Vinu, S., Biju, A., Syamaprasad, U.: Supercond. Sci. Technol. 22, 045016 (2009)
Wang, H., Serquis, A., Maiorov, B., Civale, L., Jia, Q.X., Arendt, P.N., Foltyn, S.R., Macmanus-Driscoll, J.L., Zhang, X.: J. Appl. Phys. 100, 053904 (2006)
Sarun, P.M., Vinu, S., Shabna, R., Biju, A., Syamaprasad, U.: J. Alloys Compd. 472, 13 (2009)
Shabna, R., Sarun, P.M., Vinu, S., Biju, A., Syamaprasad, U.: J. Alloys Compd. 493, 11 (2010)
Biju, A., Sarun, P.M., Aloysius, R.P., Syamaprasad, U.: J. Alloys Compd. 454, 46 (2008)
Lotgering, F.K.: J. Inorg. Nucl. Chem. 9, 113 (1959)
Gao, W., Vander-Sande, J.B.: Supercond. Sci. Technol. 5, 318 (1992)
Economy, J., Anderson, R.: Inorg. Chem. 5, 989 (1966)
Shi, L., Gu, Y., Chen, L., Yang, Z., Ma, J., Qitan, Y.: Mater. Lett. 58, 3301 (2004)
Budak, E., Bozkurt, C.: Physica B 405, 4702 (2010)
Jiang, J.: Mater. Lett. 61, 3239 (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bal, S., Dogruer, M., Yildirim, G. et al. Role of Cerium Addition on Structural and Superconducting Properties of Bi-2212 System. J Supercond Nov Magn 25, 847–856 (2012). https://doi.org/10.1007/s10948-011-1360-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-011-1360-9