Abstract
The main purpose of this research work will assess the effect of Cr addition on the properties of Bi1.8Pb0.4Sr2CaCu2O8+d superconducting ceramics. The samples are produced from industrial powders with different amounts of Cr (0, 1, 2, 3, 4, 5, 6 and 7 wt %) by sol–gel synthesis route. The influence of doping element has been determined by X-ray diffraction (XRD), scanning electron microscopy (SEM), electrical resistivity and magnetic characterizations. In particular, the eventual presence of secondary phases as a result of Cr doping on the undoped sample was investigated by using XRD. By means of SEM analysis, the Cr doping influence on the grain morphology of the samples was explored. Contrarily, the Tc,on and the Tc,off of the samples have been obtained by using electrical resistivity measurements and their behavior as a function of the Cr doping has been discussed. Finally, by performing magnetization analysis versus temperature and magnetic field, the behavior of the critical temperature Tc and of the critical current density Jc as a proportion of the Cr concentration has been discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Since their discovery, high-temperature superconductors have been classified as future materials, including those based on bismuth with higher critical density and irreversible field. These characteristics have favored these materials in various applications, such as medical diagnostics, high-speed trains (magnetic levitation), high-power transmission lines, energy storage systems, etc.” [1, 2].
From the literature of high-temperature superconductors (HTSC) [3], a numerous research works were to comprehend the characteristics of this kind of materials. Many researches are performed for the Bi(Pb)–Sr–Ca–Cu–O system (BSCOO) in order to increasing their critical current density Jc for practical field. However, these materials are sensitive to magnetic field, the critical current in the plans a-b decreases rapidly when the magnetic field increases. In this case the decreasing is more rapid whenever the field lines up with the c axis of the grains. Indeed, the low number of defects in the plans [CuO2] facilitates the movement of vortices under the effect of a Lorentz force. Until the magnetic field is perpendicular to the c axis, the vortices are anchored between the [CuO2] planes. The critical current therefore decreases more slowly with the magnetic field. This decrease is even slower when the ceramic grains are correctly oriented. It was confirmed that the BSCCO composites are appropriate for various transport applications once handled appropriately to achieve a satisfactory grain alignment [4,5,6]. Furthermore, it is pretty apparent that BSCCO superconductors have the highest values in critical current densities, critical transition temperatures (Tc), and irreversibility fields. These compounds achieved be alloys or transition metals with high electrical resistance values in the normal state, they contain flat layers of copper and oxygen atoms. These copper oxide layers carry the supercurrent, give a high Tc value, and it demonstrates greater stability in superconductors properties. The general chemical formula for Bi-based HTSC could become given by Bi2Sr2Can-1Cu2O2n+4. (Where n = 1, 2 and 3 which n represent the number of CuO2 layers and transition temperatures are 20, 95 and 110 K, respectively) [7]. The Bi-2212 component is considered a superconductor composite with more possibility to be employed in technology [8,9,10,11,12].
The chemical doping of HTSC is controllable, effective method for enhancing the mechanic, morphological and superconductivity properties of these materials.
Many chemical rare earth elements have been playing a significant effect on the nanostructure, electrical and magnetization characteristic of the superconducting materials [13,14,15,16,17,18]. The influence of excessive-doping and under-doping on Tc zero has been tested, in this regard substitutions and addition of doped element lead to stabilize the Bi-2212 phase where it exhibits the best superconducting behavior. Recently superconductivity has been discovered in chromium based compounds (CrAs, 2.2 K) with moderate magnetic moment. For this reason we have chosen Cr as dopant for the structural and magnetic properties of our superconducting material Bi2212. Moreover, Chromium metal discovered by L. N. Vauquelin in 1797 is another interesting rare earth element with exceptional magnetic properties. at room temperature, it is the only elemental solid to exhibit anti-ferromagnetic ordering. It is a transition metal, within atomic radius (140 pm) and electrical resistivity about 125 NΩ.m. Also, with the replacement of Pb in Bi increase the critical current densities [8, 19,20,21,22,23], and reduces the lattice parameter c, It influences the extra oxygen absorbed from the BiO layers. then reduces the anisotropic behavior of superconducting proprieties.
Besides chemical doping, the method and synthesis conditions must be chosen carefully. Unlike the sol–gel method, wet-chemistry synthesis techniques [24,25,26,27,28,29,30,31,32], including, solid state reaction, co-precipitation method, citrate gel process, oxidation of liquid quenched precursor alloys, and matrix reaction. Almost all of those methods have difficulties and limits that come with the actual process and the performance of the material it produces.
Sol–gel is one of the most preferred methods due to colloid solution combustion synthesis, the process synthesis on high-purity multi component, and a low temperature initiated combustion process that is found to be cost-effective for the preparation of extremely fine and homogeneous powders.
For the advancement in this area of study, Our work discusses the effect of Cr on the morphological and magnetic characteristics of Bi(Pb)-2212 composites synthesized by the sol–gel process.
2 Experimental details
2.1 Chemical synthesis
In stochiometric proportions, Bi(NO3)3, Pb(NO3)2, Sr(NO3)2, Ca(NO3)2 and Cu(NO3)2 are dissolved in distilled water.
Bi solution is amended with Cr2O3 powder dissolved in nitric acid.
Bi1.8Pb0.4Sr2CaCu2CrxO8 + d, where x = 0, 1, 2, 3, 4, 5, 6, and 7 wt%, is the nominal composition of the prepared samples. They will be labeled accordingly as Cr0, Cr1, Cr2, Cr3, Cr4, Cr5, Cr6, and Cr7.
The solution of ammonia reacts with citric acid to produce a triammonium citrate with a 2 mol/kg content.
Acrylamide CH2=CHCONH2 and N, N methylene diacrylamide CH2=CHCONHCH2NHCOCH=CH2 are combined to make organic gels and complex the Bi, Ca, Sr, and Cu cations. On a hot plate, the resulting solution is mixed, stirred, and heated to 80°-90 °C with a magnetic stirrer. Some drops of an AIBN solution (2,2′-azobis(2-methylpropionitrile). in acetone are added to speed up the gel's formation. This material is converted to Bi(Pb)-2212 by thermal treatment at 400 °C for two hours and 700 °C for six hours, respectively. In agate mortar, the powder is ground and then calcined for 12 h at 860 °C, reaching a speed of 5 °C/min.
2.2 Measurements
To perform phase analysis on the materials, X-ray diffraction (XRD) is used on a Siemens D8-Advance powder diffractometer by applying the application of CuK radiation ( λ = 1.5418 Å) with an angle increment of 0.02 in the range 2θ = 10–55°. The samples’ microstructure and surface morphology are of examined by scanning electron microscopy (SEM, JEOL 6390LV) equipped with an energy dispersive. All samples are tested for resistivity between 10 and 120 K using a typical DC four-probe method with a current of 5 mA on helium refrigerator operating in a closed cycle (Cryodine CTI-Cryogenics). The current connections and voltage are created using a silver paste in which the magnetic field is used parallel to the pellet’s surface and perpendicular to the current’s direction of flow.
Magnetization measurements were taken as a function of temperature m(T) and field m(H) utilizing a QD-PPMS equipment with a VSM option [33] It has been paid attention to reduce the residual entrapped field inside the superconducting magnet before each measurement [34, 35]. The sample was cooled in zero field to make the m(T) measurement. Then the magnetic field is activated and data acquired while increasing the temperature. After that, the sample has been cooled down again but this time in presence of field. This procedure allows to record data in Zero Field Cooling (ZFC) and Field Cooling (FC) conditions [36, 37], respectively. The m(H) measurement was obtained by first cooling the sample to 4.2 K in zero field, then ramping the field from 0 T to + 9 T, then -9 T, and finally back to + 9 T [38, 39].
The samples were examined in a dc magnetic field that was directed perpendicular to the sample's biggest face. The magnetic moment in relationship to temperature (m(T)), values has been calculated under Zero Field Cooling (ZFC)-Field Cooling (FC) conditions to determine a superconducting critical temperature Tc of our sample.
Precisely, the sample is cooled to 40 K without a magnetic field; then, the magnetic field is turned on at 0.002 T, and data are collected for temperature increase (ZFC) up to 100 K.
The sample is then cooled again, and the FC magnetic moment is obtained in the presence of a field.
3 Results and discussion
3.1 X-ray diffraction analysis
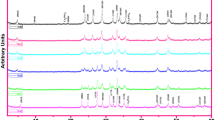
Figure 1a shows XRD pattern of the Bi(Pb)-2212 composites prepared with different contents of Cr. In addition to the lines of the main phase, ( +)-marked extra tiny lines are observed.
According to the JCPDS46-0392 file, they correspond to the Bi-2201 phase with a low Tc.
The addition of Cr affects the intensity of the peaks and the appearance of secondary peaks when the amount of Cr is increased, indicating that Cr + 3 ions are incorporated into the matrix. This result corresponds to the Cr solubility limit in Bi(Pb)-2212.
The cell characteristics are refined using the JANA 2006 program [40] and the Bbmb space group [41]. The line intensities are fitting by a pseudo-Voigt functional, the backdrop is described by 36 terms of Legendre polynomials, and the asymmetric is corrected using the Berar-Baldinazzi method.
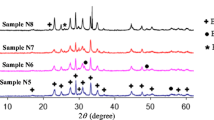
Figure 1b shows XRD data of !three samples, 0%, 3% and 7% analyzed by Rietveld refinement method. This method is based on the construction of diffraction patterns calculated according to a structural model [42]. The background was corrected using a Legendre polynomials. A peak profile function was modeled using a convolution of the pseudo–Voigt function with the asymmetry function described by Berar-Baldinozzi correction.
Table 1 presents the obtained parameters synthesized by modified sol–gel method. When there is an increase in chromium concentration, there is typically a decreasing trend in the parameters c. These behaviors can be interpreted by substitution of Cu-ion by Cr-ion in the structure, which reduces the oxygen levels in the unit cell [43, 44]. As seen from the graph no secondary phase Bi2201 appears in undoped sample, however it begins to rise as the Cr addition is increased.
By applying the appropriate standard modeling approach [45], the relative volume fractions (Table 1) of the Bi-2212 and Bi-2201 phases in each sample are determined from the intensities of the reflections:
Phase i = Bi-2212 or Bi-2201, where I2212 and I2201 are the XRD intensities of Bi-2212 and Bi-2201 phases, respectively. It can be clearly seen in Table 1 that the Bi-2201 secondary phase is more evident in doped samples and the addition of Cr decreases the intensities of Bi-2212 and increases the intensities of Bi-2201. The undoped sample has higher volume fraction of the Bi-2212 phase (90%) than other samples.
The average crystallite size (D) has calculated from Debye–Scherrer formula [46]:
where λ is the wavelength, β is the full width at half maximum FWHM and \(\theta\) is the angle of the intense peak. From Table 2 the crystallite size decrease with the increase of Cr addition.
3.2 SEM results
The contribution of Cr doping on the morphological characteristics of Bi1.8Pb0.4Sr2CaCu2CrxO8+d ceramics is studied in more detail with scanning electron microscopy, which is used to capture image of certain areas as microanalysis. Figure 2 has shown the SEM micrographs of the pure and Cr-addition samples taken at the same magnification (5000X) with a size of about 2 µm from the surface of the pellets. Doping with Cr doesn’t seem to have much of an effect on the morphology of the grain. It’s easy to see that the shape of the grains in all of all samples is very similar. The characteristic flaky grains of Bi-2212 are visible. However, it is observed that the undoped sample is denser, contain small sized grains with connectivity. This fact could induce better transport superconducting properties. So, doping with Cr worsens the surface morphology.
The addition of Chrom in the grains of the doped samples was confirmed by qualitative EDX analysis presented in the Fig. 2. Our spectrum shows the absence of the parasite phases in all samples.
3.3 DC electrical resistivity measurements
The electrical resistivity versus temperature ρ(T) of the samples, calculated using the standard four-probe dc technique between 40 and 110 K, is displayed in Fig. 3. The above data are normalized to the resistivity ρn at 110 K. All samples display a metallic-like character in the normal state. It can be easily seen that both Tc,on and Tc,off changes with doping. The values of parameters like Tc.on, Tc.off and superconducting transition width ΔTc for different amounts of Cr are listed in Table 3. Possible causes for a difference in Tc include the induction of mobile carriers, the total oxygen concentration, and the disorder in the CuO2 planes.
All samples have an almost identically narrow width of transition temperature, suggesting that there is just one superconductivity transition. Contrarily, larger transition widths are sometimes associated with a high disoriented angle, hence reducing intergranular connectivity. [47].
Hole-carrier concentrations p per Cu-ion are estimated for each sample using the Eq. (3), [7]:
while Tc,max has been assumed to be 95 K for Bi-2212 phase [26] and Tc,off values were derived in Table 1.
Doping modifies the behavior of the sample from an overdoped zone to an appropriately doped region.
The minimal hole number of 0.417 observed for the Cr3% amount indicates that it is closest to an optimal manner doped area.
Therefore, the inclusion of trivalent Cr3 + reduces the number of efficient holes.
The Tc,on of all samples correlates better with the holes ‘s concentration of CuO2 layers.
3.4 Magnetic results
All the measured curves are reported in the left panel of Fig. 4. The temperature value associated with the onset of the ZFC magnetic moment drop is defined as the Tc, and its behavior as a function of Chrome doping is reported in Table 3 and in the right panel of Fig. 4. It is visible how, starting from the undoped sample, the Tc value increases up to 4% Cr doping concentration [48]. Further increasing the doping percentage, the Tc starts to decrease. This dome shape-like behavior is usually attributed to an increase in the carrier density due to the doping. In fact, an under/optimal-doping causes a pressure induced charge transfer which enhances the Tc value in the HTSC while an over-doping determines an increase in coulomb correlation which causes a lowering of Tc values [49, 50].
In Fig. 5, the m(H) curves for various samples have been presented. The existence of flux pinning centers is visible looking at the m(H) shape [51]. Moreover, possibly surface barriers are present when the superconducting hysteresis loops are not symmetrical [52]. Understanding how the Cr doping effects on the transport capabilities of the sample, the Bean critical state approach has been applied to determine the critical current density Jc from m(H) curves [53,54,55] Since Jc is dependent on the width of the hysteresis loop, the formula (4) for calculating it in slab geometry is as follows:
where \(\Delta M={M}_{\mathrm{dn}}-{M}_{\mathrm{up}}\), \({M}_{\mathrm{dn}}\) and \({M}_{\mathrm{up}}\) refers for magnetization, which can be measured with decreasing and increasing applied field, accordingly, a (cm) and b (cm) are the sample dimensions \(\left(b<a\right)\).
It is evident that the general shape of Jc is typical for type-II superconductors (Fig. 6, left panel) without the presence of peculiar phenomena such as second magnetization peak effect or multidomain Jc behavior [56,57,58,59] very often due to pinning crossover and/or vortex dynamics which can be efficiently studied by using the multiharmonic ac magnetic susceptibility method [60,61,62]. By extracting the value of Jc at fixed fields from the Jc(H) curves reported in the left panel of Fig. 6, the Jc can be plotted as a function of the Cr doping concentration for various magnetic fields (see right panel of Fig. 6). In this graph it is visible how the Jc drops at 1% Cr doping for all the fields. Then, increasing the Cr doping to 2%, the Jc starts to increase for all the fields until it reaches its maximum value for a Cr doping concentration equal to 3% for all the fields. This development is a result of the decrease in intrinsic anisotropy and to the fact that the addition can provide new efficient pinning centers at highest fields [45]. On the other hand, for higher amount of Cr, the Jc values start to decrease probably due to an excessive number of impurities which locally destroys the superconductivity phase of the sample so worsening its current transport capabilities.
Critical current density Jc as a function of magnetic field (left) extracted from the m(H) curves in Fig. 5. In the right panel the superconducting critical current for different magnetic fields is plotted as a function of the Chrome doping
4 Conclusion
X-ray diffraction (XRD), scanning electron microscopy (SEM), electrical resistivity, and magnetic characterizations were performed on various Bi1.8Pb0.4Sr2CaCu2CrxO8 + d samples with x = 0, 1, 2, 3, 4, 5, 6, and 7 wt%.
We discovered other smaller lines attributable to the Bi-2201 low Tc phase appearing in the doped samples using X-ray diffraction analysis, in addition to the main 2212 phase lines.
This result shows that the presence of Cr inhibits the formation of the Bi2212 phase while increasing the presence of the Bi-2201 secondary phase. Except for a slight effect on grain size, the SEM analysis revealed that the Cr doping has little effect on the surface morphology of the sample.
Studies of DC electrical resistivity versus temperature revealed that Cr doping altered both Tc,on and Tc,off of the samples.
The Cr6% and Cr2% have the highest values for Tc,on and Tc,off, respectively.
The critical temperature Tc and critical current density Jc as a function of doping concentration were calculated using dc magnetic measurements.
The highest value for Tc was obtained for the Cr4% doped sample due to a possible increase in carrier density with doping.
We also discovered that an initial Cr addition (up to 3%) can provide new favorable pinning centers, improving the pinning landscape of the samples and leading to an increase in Jc.
By continuing to add Cr% to the compound, however, the Jc begins to decrease, most likely due to an excess of impurities, which worsens the compounds' superconductivity phase.
Data availability
The authors can be reached for a simple suggestion on the datasets produced and/or examined since the current work.
References
R. Foltyn, L. Civale, J.L. MacManus-Driscoll, Q.X. Jia, B. Maiorov, H. Wang, M. Maley, Materials science challenges for high-temperature superconducting wireS. Nat. Mater. 6, 631–642 (2007)
W.H. Fietz, C. Barth, S. Drotziger, W. Goldacker, R. Heller, S.I. Schlachter, K.P. Weiss, prospects of high temperature superconductors for fusion magnets and power applications. Fusion Eng. Des. 88(6–8), 440–445 (2013)
J.G. Bednorz, K.A. Müller, Possible high Tc superconductivity in the Ba-La-Cu-O system. Zeitschrift für Phys. B Condens. Matter. 64, 189–193 (1986). https://doi.org/10.1007/BF01303701
B. Seeber, Handbook of Applied Superconductivity (IOP Publishing Ltd, Bristol, 1998)
M. Noe, K.P. Juengst, F.N. Werfel, S. Elschner, J. Bock, F. Breuer, R. Kreutz, Testing bulk HTS modules for resistive superconducting fault current limiters. IEEE Trans. Appl. Supercond. 13, 1976–1979 (2003). https://doi.org/10.1109/TASC.2003.812953
S. Marinel, D. Bourgault, O. Belmont, A. Sotelo, G. Desgardin, Microstructure and transport properties of YBCO zone melted samples processed in a microwave cavity and infra-red furnace. Phys. C Supercond. its Appl. 315, 205–214 (1999). https://doi.org/10.1016/S0921-4534(99)00202-6
F. Ben Azzouz, A. M’Chirgui, B. Yangui, C. Boulesteix, M. Ben Salem, Synthesis, microstructural evolution and the role of substantial addition of PbO during the final processing of (Bi, Pb)-2223 superconductors. Phys. C Supercond. its Appl. 356, 83–96 (2001). https://doi.org/10.1016/S0921-4534(01)00124-1
N. Musolino, S. Bals, G. Van Tendeloo, N. Clayton, E. Walker, R. Flükiger, Modulation-free phase in heavily Pb-doped (Bi, Pb)2212 crystals. Phys. C Supercond. its Appl. 399, 1–7 (2003). https://doi.org/10.1016/S0921-4534(03)01324-8
Y. Li, S. Kaviraj, A. Berenov, G.K. Perkins, J. Driscoll, A.D. Caplin, G.H. Cao, Q.Z. Ma, B. Wang, L. Wei, Z.X. Zhao, Enhancement of critical current density of (Pb, Sn)-doped Bi-2212 superconductors at high temperature. Phys. C Supercond. its Appl. 355, 51–58 (2001). https://doi.org/10.1016/S0921-4534(00)01770-6
P.M. Sarun, S. Vinu, R. Shabna, A. Biju, U. Syamaprasad, Highly enhanced superconducting properties of Eu-doped (Bi, Pb)-2212. Mater. Lett. 62, 2725–2728 (2008). https://doi.org/10.1016/j.matlet.2008.01.026
S. Vinu, P.M. Sarun, R. Shabna, A. Biju, U. Syamaprasad, Microstructure and transport properties of Bi1.6Pb0.5Sr2-xLuxCa1.1Cu2.1O8+δ superconductor. Mater. Chem. Phys. 119, 135–139 (2010). https://doi.org/10.1016/j.matchemphys.2009.08.049
S. Uthayakumar, E. Srinivasan, R. Jayavel, C. Subramanian, Substitutional effect of Mn on floating zone growth Bi-2212 bulk textured crystals. Phys. C Supercond. its Appl. 383, 122–126 (2002). https://doi.org/10.1016/S0921-4534(02)01264-9
S. Bal, M. Dogruer, G. Yildirim, A. Varilci, C. Terzioglu, Y. Zalaoglu, Role of cerium addition on structural and superconducting properties of Bi-2212 system. J. Supercond. Nov. Magn. 25, 847–856 (2012). https://doi.org/10.1007/s10948-011-1360-9
O. Ozturk, E. Asikuzun, G. Yildirim, The role of Lu doping on microstructural and superconducting properties of Bi2Sr2CaLuxCu2Oy superconducting system. J. Mater. Sci. Mater. Electron. 24, 1274–1281 (2013). https://doi.org/10.1007/s10854-012-0918-z
K. Belala, A. Galluzzi, M.F. Mosbah, M. Polichetti, Transport and magnetic properties of Bi(Pb)2212 superconducting ceramics doped by low rate of potassium. Mater. Sci. 39, 15–23 (2021). https://doi.org/10.2478/msp-2021-0005
O. Ozturk, E. Asikuzun, M. Coskunyurek, N. Soylu, A. Hancerliogullari, A. Varilci, C. Terzioglu, The effect of Nd2O3 addition on superconducting and structural properties and activation energy calculation of Bi-2212 superconducting system. J. Mater. Sci. Mater. Electron. 25, 444–453 (2014). https://doi.org/10.1007/s10854-013-1608-1
E. Asikuzun, O. Ozturk, H.A. Cetinkara, G. Yildirim, A. Varilci, M. Ylmazlar, C. Terzioglu, Vickers hardness measurements and some physical properties of Pr2O 3 doped Bi-2212 superconductors. J. Mater. Sci. Mater. Electron. 23, 1001–1010 (2012). https://doi.org/10.1007/s10854-011-0537-0
S. Menassel, M.F. Mosbah, Y. Boudjadja, S.P. Altintas, A. Varilci, C. Terzioglu, Effect Y substitution on the microstructure, transport and magnetic proprieties of Bi2Sr2Ca1Cu2O8+δ superconducting ceramics. Mater. Sci. Pol. 34, 582–590 (2016). https://doi.org/10.1515/msp-2016-0077
I. Chong, Z. Hiroi, M. Izumi, J. Shimoyama, Y. Nakayama, K. Kishio, T. Terashima, Y. Bando, M. Takano, High critical-current density in the heavily Pb-doped Bi2Sr2CaCu2O(8+δ) superconductor: Generation of efficient pinning centers. Science (80-. ) 276, 770–773 (1997). https://doi.org/10.1126/science.276.5313.770
R. Funahashi, I. Matsubara, K. Ueno, K. Mizuno, Isotropic pinning in heavily Pb-doped Bi-2212/Ag tapes. Phys. C Supercond. its Appl. 315, 247–253 (1999). https://doi.org/10.1016/S0921-4534(99)00237-3
L. Shi, Q. Dong, Y. Zhang, Effect of Pb-doping and annealing on the structure and Tc of Bi-2212 phase superconductor. Phys. C Supercond. its Appl. 341–348, 649–650 (2000). https://doi.org/10.1016/S0921-4534(00)00632-8
D.M. Pooke, G.V.M. Williams, Oxygen loading in (Bi, Pb)-2212 and -2223 materials. Phys. C Supercond. its Appl. 354, 396–400 (2001). https://doi.org/10.1016/S0921-4534(01)00107-1
F. Jean, G. Collin, M. Andrieux, N. Blanchard, A. Forget, S. Rousseau, J.F. Marucco, Oxygen excess in Bi-2212: study of a Pb-substituted compound. Phys. C Supercond. its Appl. 384, 345–350 (2003). https://doi.org/10.1016/S0921-4534(02)01969-X
Raheleh Yousefi Seyede, Sobhani Azam, Salavati-Niasari. Masoud, A new nanocomposite superionic system (CdHgI4/HgI2):Synthesis, characterization and experimental investigation. Adv. Powder Technol. 28, 1258–1262 (2017). https://doi.org/10.1016/j.apt.2017.02.013
M.R. Presland, J.L. Tallon, R.G. Buckley, R.S. Liu, N.E. Flower, General trends in oxygen stoichiometry effects on Tc in Bi and Tl superconductors. Phys. C Supercond. its Appl. 176, 95–105 (1991). https://doi.org/10.1016/0921-4534(91)90700-9
H.R. Zhuang, H. Kozuka, S. Sakka, Preparation of superconducting Bi-Sr-Ca-Cu-O ceramics by the sol-gel method. J. Mater. Sci. 25, 4762–4766 (1990). https://doi.org/10.1007/BF01129938
N. Hara, R. Ogawa, Y. Kawate, T. Tateishi, Preparation of Bi based high-Tc superconductors containing Pb and Sb by the sol-gel method. J. Mater. Res. 7, 292–298 (1992). https://doi.org/10.1557/JMR.1992.0292
K. Ma, A.C. Pierre, Sol-gel processing of high-Tc superconductors in the Bi-(Pb)-Sr-Ca-Cu-O system. J. Mater. Res. 7, 1328–1335 (1992). https://doi.org/10.1557/JMR.1992.1328
B.S. Ahn, Synthesis of BiSrCaCu(Ni)O ceramics from the gel precursors and the effect of Ni substitution. Bull. Korean Chem. Soc. 23, 1304–1308 (2002). https://doi.org/10.5012/bkcs.2002.23.9.1304
F.H. Chen, H.S. Koo, T.Y. Tseng, Synthesis of high-Tc superconducting Bi-Pb Sr-Ca-Cu-O ceramics prepared by an ultrastructure processing via the oxalate route. J. Mater. Sci. 25, 3338–3346 (1990). https://doi.org/10.1007/BF00587696
T.S. Hen, J.R. Chen, T.Y. Tseng, Preparation of bi0.7pb0.3sr1.0ca1.0cu1.8oy high-tc superconductor by the citrate method. Jpn. J. Appl. Phys. 29, 652–655 (1990). https://doi.org/10.1143/JJAP.29.652
T.M. Chen, Y.H. Hu, Polymeric precursors for the preparation of Bi1.5Pb0.5Sr2Ca2Cu3Ox. J. Solid State Chem. 97, 124–130 (1992). https://doi.org/10.1016/0022-4596(92)90016-O
Raheleh Yousefi Seyede, Abbas Alshamsi Hassan, Omid Amiri, Salavati-Niasari. Masoud, Synthesis, characterization and application of Co/Co3O4 nanocomposites as an effective photocatalyst for discoloration of organic dye contaminants in wastewater and antibacterial properties. J. Mol Liq. 337, 116405 (2021). https://doi.org/10.1016/j.molliq.2021.116405
A. Galluzzi, K. Buchkov, E. Nazarova, V. Tomov, G. Grimaldi, A. Leo, S. Pace, M. Polichetti, Transport properties and high upper critical field of a Fe(Se, Te) iron based superconductor. Eur. Phys. J. Spec. Top. (2019). https://doi.org/10.1140/epjst/e2019-800169-5
A. Galluzzi, K.M. Buchkov, E. Nazarova, V. Tomov, G. Grimaldi, A. Leo, S. Pace, M. Polichetti, Pinning energy and anisotropy properties of a Fe(Se, Te) iron based superconductor. Nanotechnology. 30, 254001 (2019). https://doi.org/10.1088/1361-6528/ab0c23
A. Galluzzi, D. Mancusi, C. Cirillo, C. Attanasio, S. Pace, M. Polichetti, Determination of the transition temperature of a weak ferromagnetic thin film by means of an evolution of the method based on the Arrott Plots. J. Supercond. Nov. Magn. 31, 1127–1132 (2018). https://doi.org/10.1007/s10948-017-4281-4
A. Galluzzi, A. Nigro, R. Fittipaldi, A. Guarino, S. Pace, M. Polichetti, DC magnetic characterization and pinning analysis on Nd1.85Ce0.15CuO4 cuprate superconductor. J. Magn. Magn. Mater. 475, 125–129 (2019). https://doi.org/10.1016/J.JMMM.2018.11.119
Galluzzi, A., Buchkov, K., Tomov, V., Nazarova, E., Leo, A., Grimaldi, G., Nigro, A., Pace, S., Polichetti, M.: Second Magnetization Peak Effect in a Fe(Se,Te) iron based superconductor. In: Journal of Physics: Conference Series. p. 012012. IOP Publishing (2019)
M. Polichetti, A. Galluzzi, K. Buchkov, V. Tomov, E. Nazarova, A. Leo, G. Grimaldi, S. Pace, A precursor mechanism triggering the second magnetization peak phenomenon in superconducting materials. Sci. Rep. 11, 7247 (2021). https://doi.org/10.1038/s41598-021-86728-8
V. Petrícek, M. Dušek, L. Palatinus, Crystallographic computing system JANA2006: general features. Zeitschrift fur Krist. 229, 345–352 (2014). https://doi.org/10.1515/zkri-2014-1737
A. Amira, Y. Boudjadja, A. Saoudel, A. Varilci, M. Akdogan, C. Terzioglu, M.F. Mosbah, Effect of doping by low content of yttrium at Ca and Sr sites of Bi(Pb)-2212 superconducting ceramics. Phys. B Condens. Matter. 406, 1022–1027 (2011). https://doi.org/10.1016/j.physb.2010.12.052
H.M. Rietveld, J. Appl. Cryst. 2(2), 65–71 (1969)
A. Naseem, Shakeel, Khan, Effect of (Mn-Co) co-doping on the structural, morphological, optical, photoluminescence and electrical properties of SnO2. J. Alloy. Compd. 720, 502–509 (2017). https://doi.org/10.1016/j.jallcom.2017.05.293
A. Naseem, Shakeel, Khan, Mohd, Mohsin Nizam Ansari, Optical, dielectric and magnetic properties of Mn doped SnO2 diluted magnetic semiconductors. Ceram. Int. 44, 15972–15980 (2018). https://doi.org/10.1016/j.ceramint.2018.06.024
B. Jayaram, P.C. Lanchester, M.T. Weller, Localization and interaction effects during superconductor-insulator transition of Bi2Sr2Ca1-xGdxCu2O8+d. Phys. Rev. B. 43, 5444–5450 (1991). https://doi.org/10.1103/PhysRevB.43.5444
B.D. Cullity, Element of X-ray differaction (Addition-Wesley, Boston, 1978)
Y. Boudjadja, A. Amira, N. Mahamdioua, A. Saoudel, S. Menassel, A. Varilci, C. Terzioglu, S.P. Altintas, Microstructural and magneto-transport properties of Bi1.6Pb0.4Sr2Ca1−xGdxCu2O8+δ superconducting ceramics. Phys. B Condens. Matter. 505, 68–73 (2017). https://doi.org/10.1016/j.physb.2016.10.035
C. Böhmer et al., Supercond. Sci.Technol. 10 (1997)].[Y. Enomoto et al.J. Phys. Condens.Matter 9 (1997)]).
O. Kraut, C. Meingast, G. Bräuchle, H. Claus, A. Erb, G. Müller-Vogt, H. Wühl, Uniaxial pressure dependence of Tc of untwinned YBa2Cu3Ox single crystals for x=6.5–7. Phys. C Supercond. its Appl. 205, 139–146 (1993). https://doi.org/10.1016/0921-4534(93)90180-X
H. Claus, M. Braun, A. Erb, K. Röhberg, B. Runtsch, H. Wühl, G. Bräuchle, P. Schweib, G. Müller-Vogt, Löhneysen, H. v.: The “90 K” plateau of oxygen deficient YBa2Cu3O7-δ single crystals. Phys. C Supercond. Appl. 198, 42–46 (1992). https://doi.org/10.1016/0921-4534(92)90263-C
D. Dew-Hughes, Flux pinning mechanisms in type II superconductors. Philos. Mag. 30, 293–305 (1974). https://doi.org/10.1080/14786439808206556
L. Zhang, Q. Qiao, X.B. Xu, Y.L. Jiao, L. Xiao, S.Y. Ding, X.L. Wang, Surface barrier and bulk pinning in MTG YBaCuO. Phys. C Supercond. its Appl. 445–448, 236–239 (2006). https://doi.org/10.1016/j.physc.2006.04.008
C.P. Bean, Magnetization of hard superconductors. Phys. Rev. Lett. 8, 250–253 (1962). https://doi.org/10.1103/PhysRevLett.8.250
C.P. Bean, Magnetization of high-field superconductors. Rev. Mod. Phys. 36, 31–39 (1964). https://doi.org/10.1103/RevModPhys.36.31
A. Umezawa, G.-W. Crabtree, J.-Z. Liu, H.-W. Weber, W.-K. Kwok, L.-H. Nurez, T.J. Morun, C.-H. Sowers, H. Claus, Enhanced critical magnetization currents due to fast neutron irradiation in single-crystal YBa2Cu3O7+δ. Phys. Rev. B 36, 7151–7153 (1987)
W. Zhou, X. Xing, W. Wu, H. Zhao, Z. Shi, Second magnetization peak effect, vortex dynamics, and flux pinning in 112-type superconductor Ca0.8La0.2Fe1-xCoxAs2. Sci. Rep. 6, 22278 (2016). https://doi.org/10.1038/srep22278
A. Galluzzi, K. Buchkov, V. Tomov, E. Nazarova, A. Leo, G. Grimaldi, A. Nigro, S. Pace, M. Polichetti, Evidence of pinning crossover and the role of twin boundaries in the peak effect in FeSeTe iron based superconductor. Supercond. Sci. Technol. 31, 015014 (2018). https://doi.org/10.1088/1361-6668/aa9802
Miu, L., Ionescu, A.M., Miu, D., Ivan, I., Crisan, A.: Behavior of the Second Magnetization Peak in Self-nanostructured La2–xSrxCuO4 Single Crystals. Presented at the (2017)
A. Galluzzi, M. Polichetti, K. Buchkov, E. Nazarova, D. Mancusi, S. Pace, Evaluation of the intragrain critical current density in a multidomain FeSe crystal by means of dc magnetic measurements. Supercond. Sci. Technol. 28, 115005 (2015). https://doi.org/10.1088/0953-2048/28/11/115005
M.G. Adesso, C. Senatore, M. Polichetti, S. Pace, Harmonics of the AC susceptibility as probes to differentiate the various creep models. Phys. C Supercond. 404, 289–292 (2004). https://doi.org/10.1016/j.physc.2003.09.096
M.G. Adesso, M. Polichetti, S. Pace, Harmonics of the AC susceptibility for the study of I-V curves in melt grown YBCO. Phys. C Supercond. Appl. 401, 196–200 (2004). https://doi.org/10.1016/j.physc.2003.09.036
C. Senatore, M. Polichetti, D. Zola, T.D. Matteo, G. Giunchi, S. Pace, Vortex dynamics and pinning properties analysis of MgB 2 bulk samples by ac susceptibility measurements. Supercond. Sci. Technol. 16, 183–187 (2003). https://doi.org/10.1088/0953-2048/16/2/310
Acknowledgements
The authors gratefully would like to thank Dr. Sevgi Polat Altintas, Pr. Cabir Terzioglu (Abant Izzet Baysel university, Bolu-Turkey), Pr. Massimiliano Polichetti and Dr. Armando. Galluzzi (Salerno university- Italy) for their invaluable supports.
Funding
The authors of this publication note that they were not provided with any funding, grants, or other support during its realization.
Author information
Authors and Affiliations
Contributions
SPA and CT analyzed the XRD, SEM and resistivity results. AG and MP contributed to the magnetic analysis. YB commented on The first draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial statement or other interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Menassel, S., Galluzzi, A., Boudjadja, Y. et al. Investigation of Cr addition effect on structural, morphological, electrical and magnetic properties of Bi(Pb)-2212 superconductors. J Mater Sci: Mater Electron 34, 1145 (2023). https://doi.org/10.1007/s10854-023-10524-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-10524-5