Abstract
We conducted a paleolimnological study to examine how changes in human activity interacted with climate to influence productivity and biodiversity over the past millennium in oligotrophic Lake Atnsjøen, SE Norway. The study included analyses of sediment geochemistry, subfossil diatoms and cladocerans, and macrofossils. Results were compared with the historical record of human activities in the catchment, pollen analysis and paleoclimate inferences from the lake. During the first 750 years of the record (1000–1750 CE), a time of relatively low human activity, lake productivity and biodiversity were strongly related to climate. During the Little Ice Age (1550–1800 CE), lake productivity and diatom diversity were constrained by cold climate. A century of climate warming (1780–1880 CE) initiated an increase in productivity. Accelerated human settlement after 1850 CE, however, had an even stronger impact on productivity, mediated by increased agriculture and/or forestry, which led to greater nutrient loading of the lake. Similarly, diatoms in the lake responded to the rise in temperature, but increasing human activity also had a moderate impact on the diatom community, which displayed weak signs of nutrient enrichment. From 1980 to 1990 CE onwards, lake productivity declined as a consequence of a recent decrease in human activity and changing land use. The human-induced increase in lake productivity starting ca. 1850 CE propagated through the food web and increased consumer productivity, as reflected by greater accumulation rates of cladocerans, trichopterans and turbellarians. The cladoceran community was likely under top-down control of fish, as indicated by changes in size structure and diversity. Our study showed that increasing human activity during the settlement period had a stronger impact on lake productivity than did climate. Furthermore, the slight human-mediated increase in nutrient loading had different impacts on productivity and biodiversity in the study lake. This study demonstrates that even relatively small changes in human activities in watersheds can have measurable impacts on nutrient-poor lakes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic forcing is increasingly impacting freshwater ecosystems (Dudgeon et al. 2006). At the watershed scale, this impact includes human activities like agriculture, forestry, urban development and fish stocking (Anderson et al. 1995; Alric et al. 2013; Bragée et al. 2013; Berthon et al. 2014). These activities affect productivity and biodiversity of freshwater communities (McQueen et al. 1986; Dodson et al. 2000; Dong et al. 2012a; Alric et al. 2013; Jensen et al. 2013) and often lead to poor water quality, ultimately threatening important ecosystem services provided by freshwater environments. Management of the freshwater environment to mitigate negative human impacts requires an understanding of how effects are related to the level of disturbance (i.e. low vs. high disturbance) and how the whole ecosystem is affected.
Few comprehensive studies of anthropogenic forcing of lake ecosystems have focused on both productivity and biodiversity, the latter term used here in a broad “compositional” sense to refer to the variety of life, with organisms aggregated as species and communities (Callicott et al. 1999), and encompassing several trophic levels. The few studies with such a broad focus consider intermediate to high levels of disturbance (Jeppesen et al. 2000). The high number of oligotrophic and ultra-oligotrophic lakes found in remote regions with low human population densities, such as large parts of Scandinavia and North America (Hessen et al. 1995; Clark et al. 2010), are often perceived as near-pristine (undisturbed) freshwaters. There are, however, a number of studies that suggest even low levels of human activity have an impact on lake ecosystems (Perren et al. 2012; Wolfe et al. 2013; Neil and Gajewski 2017). The ecological response of an oligotrophic lake to increased human activity depends on the range and magnitude of activity change (Colby et al. 1972). Increased research on the consequences of low-level disturbance in these lakes will not only improve our understanding of human impacts on lake ecosystems, but will also have implications for the conceptual framework in management of these lakes, which are often viewed as reference sites from a monitoring perspective.
Climate change is increasingly threatening the biosphere (IPCC 2013). Climate influences aquatic ecosystems directly through changes in temperature, precipitation and wind exposure, but it may also interact with local anthropogenic stressors to affect lake ecosystems indirectly (Whitehead et al. 2009). Studies have shown that lake responses to climate change may depend on nutrient loading and vice versa. For example, climate warming may have stronger effects on temperate lakes that receive higher nutrient loads than on nutrient-poor lakes (Alric et al. 2013). Likewise, the response to increased nutrient loads may depend on temperature; e.g. lake responses to increasing nutrient loads at lower temperatures may be damped because of temperature constraints on biological processes and communities. Increasing human pressure on lake ecosystems, in combination with climate warming in recent centuries (Dong et al. 2012b; Cao et al. 2014), underpins the need to consider climate effects when striving to understand anthropogenic impacts on lake ecosystems.
The role of climate as a driver of natural biological communities has been investigated in studies that relate changes in biological communities in remote arctic and alpine lakes to instrumental climate records (Korhola et al. 2002). Paleolimnology has become an important tool in the assessment of human impacts (e.g. eutrophication) on lakes (Davidson and Jeppesen 2013). The combined effects of climate and human forcing of ecosystem structure and/or function were addressed in several paleolimnological studies in densely populated areas (Perga et al. 2010; Dong et al. 2012a; McGowan et al. 2012; Alric et al. 2013; Berthon et al. 2014; Cao et al. 2014). Few studies have addressed this issue in nutrient-poor lakes under low-intensity human impact.
This study was undertaken to assess how productivity and biodiversity in a nutrient-poor lake were affected by human activity in the catchment and by climate during the last millennium. We used paleolimnological methods and historical documentation to decipher the climate-human-lake ecosystem relationships. By focusing on both primary and secondary producers we were able to examine how different trophic levels responded to external stressors. We measured proxies for productivity (fluxes of trichopterans, turbellarians, cladocerans and organic carbon) and biodiversity (species richness and community composition of diatoms and cladocerans) to test if the two responded differently to changing human activity and climate.
Materials and methods
Study site and catchment history
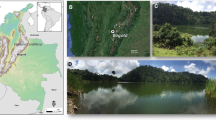
Oligotrophic Lake Atnsjøen (61°52′51 N, 10°09′55 E) is situated in southeast Norway, in the municipality of Stor-Elvdal. The lake is located at 701 m a.s.l. and has a surface area of 4.8 km2, maximum depth of 80 m, mean depth of 35.4 m and a water retention time of 6 months (Fig. 1). The catchment area is 457 km2, most of which (85%) is above tree line (~ 1000 m a.s.l.). High-elevation flora consists of high- and low-alpine tundra, whereas pine (Pinus sylvestris) and birch (Betula pubescens) dominate below tree line. The catchment area consists mainly of feldspar quartzite, with locally large deposits of Quaternary and fluvial materials. The area around Lake Atnsjøen has a continental climate with average annual temperature and precipitation of 0.7 °C and 555 mm, respectively (Nordli and Grimenes 2004). Ice cover on the lake lasts from late November to late May. The Atna watershed, including Lake Atnsjøen, remains relatively unaffected by human activities because of its remote location and because a large part of the catchment lies within the Rondane National Park.
There are indications of early, low-level human activity in the area around Lake Atnsjøen during the last millennium (Mikkelsen 1980). Human development around Lake Atnsjøen from Medieval times and onwards is relatively well documented compared to what is known about many other remote areas in Norway, because the Atnsjøen region was repopulated relatively late after the Black Death (1348–1349 CE). Thus, it is possible to track settlement changes over time using historical records/sources (Fig. 2, Dahl 1960; Mikkelsen 1980). To verify the development of human presence/activity in the area and to gain further insight into early human presence before and after the Black Death, historical records were supplemented with pollen analysis from Lake Atnsjøen, using human pollen indicators (HPI) and the ratio of arboreal pollen (AP, arboreal pollen: trees and shrubs) to nonarboreal pollen (NAP, non-arboreal pollen: herbs, excluding telmatic and aquatic plants), AP/NAP, as an indicator of landscape openness (Fig. 2, Zawiska et al. 2017).
Historical records of population in Folldal, the neighboring community to the area around Lake Atnsjøen), permanent settlements and “saeters” in the catchment of Lake Atnsjøen, human pollen indicators (sum of Rumex acetosa/acetosella type, Plantago lanceolata, Plantago media/major type, Anthemis type, Artemisia, Chenopodiaceae, Urtica, Cannabis sativa type, Secale cereale and Cerealia undiff.) and ratio of arboreal pollen (AP) to non-arboreal pollen (NAP) in the Lake Atnsjøen sediment core. Data on Folldal population are from A. Alander (pers. commun.) and relate to the area of the current Folldal municipality. Data on permanent settlements and “seaters” come from Dahl (1960) and H.E. Nesset (pers. commun.). Pollen data are from Zawiska et al. (2017). Periods of low (1000–1750 CE) and high (1750 CE - present) human activity are separated by a dashed line. Periods of cooling according to Zawiska et al. (2017) are highlighted by grey shading
Human activity around Lake Atnsjøen during the last millennium can be divided roughly into two periods: (1) 1000–1750 CE, (2) 1750 CE—present (Table 1). The first permanent settlement in the catchment after the Black Death was established in 1756 CE (Table 1, Fig. 2, Dahl 1960). From that time onwards, human presence in the area increased until the first half of the twentieth century, as estimated from settlements and summer farms in the catchment, and from the population density in Folldal, the neighboring community northwest of the Atna area (Fig. 2). Around 1950 CE, population in the area decreased (Fig. 2, Brænd 1989). A number of events during the twentieth century, e.g. changing agricultural practices, adoption of chemical fertilizers, industrialization/mechanization of agriculture and forestry intensified anthropogenic pressures on the lake and likely helped maintain higher pressures even after the population/number of farms/settlements began to decrease around 1950 CE (Table 1). Pollen analysis supports the historical record on human activity. The HPI was generally lower before than after 1750 CE, the latter being the period of higher human activity. Second, many events in the historical record are verified/supported by the pollen analysis, e.g. the increase in the settlement and agricultural activity, and changes in agricultural practices between 1900 and 1950 CE (Table 1).
Population density in neighboring Folldal municipality varied between 0 and 1.86 inhabitants km−2 (1950–1960 CE). Exact population densities in the Lake Atnsjøen catchment are not known, but assuming an average of six persons per household (H.E. Nesset pers. commun.), population densities in the catchment varied between 0 and 0.46 inhabitants km−2 (1700–1950 CE). These densities are low in Norwegian, European and North American contexts (Goldewijk 2005; Goldewijk et al. 2010; Statistics Norway 2018) and highlight the low human pressure on the lake in a broad perspective, even during the period of highest population density in the catchment.
Sampling and dating
We collected a core in the southeastern end of the lake, relatively close to the outlet, at a water depth of 20 m (Fig. 1). Sediment in the western, deeper part of the lake consists of relatively coarse (gravelly) clastic material that originated from fluvial inputs to the water body. In addition, pelagic Eubosmina constitutes more than 96% of the cladocaran remains in the sediment from that part of the basin (G. Halvorsen, pers. commun.). We chose a shallower sampling site in the eastern end of the lake to avoid the gravel layer and obtain a longer core that could also capture the littoral signal. A 34-cm-long sediment core was retrieved using a KC-Denmark Kajak-type gravity corer. The core was sectioned in the field into 1-cm layers, further separated into subsamples for different analyses, and stored at 5 °C.
Details of the core chronology were published in Zawiska et al. (2017). In short, the age-depth model for the core was based on 210Pb dating of topmost sediments, AMS 14C dating of terrestrial macrofossils in the lower part of the core, and pollen. Activity of total 210Pb was determined indirectly using alpha spectrometry, described by Tylmann et al. (2016). Ages for topmost sediment layers were calculated with the CF:CS (Constant Flux:Constant Sedimentation) model. Only one radiocarbon date was obtained (32 cm, 14C 1200 ± 30 BP) because of a lack of preserved terrestrial macrofossils in the sediments. Therefore, simple linear extrapolation, based on mean mass accumulation rate from the CF:CS model (0.013 g cm−2 yr−1), was used to calculate sediment ages at different depths. Additional time control from pollen data came from a sharp decline in HPI related to the Black Death plague, which peaked in Norway about 1350 CE, killing 50% of the human population (Oeding 1990). Comparison of extrapolated 210Pb dates, the AMS 14C date, and pollen data suggested that the radiocarbon date was too old by ~ 150 years. Hence, the combined 210Pb and pollen data were used for the final age-depth model (Electronic Supplementary Material [ESM] Fig. S1).
Diatom, cladocera and macrofossil analysis
Diatom slides were prepared using sub-samples from 1-cm intervals along the length of the sediment core. Organic matter and carbonates were removed from samples with H2O2 and HCl, respectively (Battarbee 1986), and superfluous mineral material was eliminated before preparing diatoms slides. The diatom suspension was dried on a cover slip and mounted with Naphrax® for microscopic analysis. At least 300 diatom valves on each slide were identified and counted according to Schrader and Gersonde (1978) and taxonomic determination was based on Krammer and Lange-Bertalot (1986, 1988, 1991a, b).
One-cm3 sediment subsamples were prepared for Cladocera analysis following standard procedures (Frey 1986). A volume of 0.1 ml from each sample was used to prepare slides that were examined by microscopy. All skeletal elements (head shields, shells and post-abdomens) were used for identification of cladoceran taxa, following Szeroczyńska and Sarmaja-Korjonen (2007) and at least 100 individuals were counted per sample (Kurek et al. 2010). Cladoceran densities by volume were converted to density per g dry weight (DW), and species percentages were calculated. We calculated the fraction of large-bodied species according to Moss et al. (2003, Daphnia longispina and Eurycercus lamellatus) as a proxy for the size structure of the cladoceran community. We used cladoceran flux as a proxy for zooplankton/cladoceran productivity. Total cladoceran flux for the Lake Atnsjøen sediment core was presented by Zawiska et al. (2017). In the current study we present the fluxes of pelagic and littoral cladocerans separately to examine if productivity of the pelagic and littoral zones responded differently to human impact. Cladoceran flux was calculated by multiplying pelagic or littoral cladoceran density by sediment dry density and dividing by the number of years represented by the sample.
Samples for macrofossil analysis were prepared according to Birks (2007). Identification of fossil remains was based on Katz et al. (1977), Birks (2007) and Velichkevich and Zastawniak (2008) and a reference collection (Institute of Geography, Polish Academy of Sciences). All macrofossil counts were standardized as numbers of fossils per 50 cm3 and converted to density per g dry weight (DW). Here we present the results of trichopteran and turbellarian remains. Trichopteran and turbellarian flux were calculated by multiplying trichopteran or turbellarian density by sediment dry density and dividing by the number of years represented by the sample.
Geochemical analysis and climate reconstruction
For C and N analysis, sediment samples were first oven-dried at 50 °C and powdered in a Pulverizette 2 agate mill (Fritsch, Germany). Total concentrations of N and C, denoted as TN and TC, respectively, were determined using a VarioMax CNS analyzer (Elementar, Germany). To determine the content of total organic carbon (TOC), samples were first decarbonated with 1 M HCl. After the reaction was complete, the residue was air-dried and then oven-dried at 50 °C and re-analyzed. The quality of measurements was controlled with certified reference materials provided by Elementar (Sulfadiazine, chalky soil (BN230229), sandy soil (BN225497) and peaty soil (BN230227)). Recovery was always greater than 95% for both elements. TN and TOC contents were expressed as wt.%. To assess lake paleoproductivity, the TOC flux (mg C cm−2 yr−1) (Routh et al. 2009; Wang et al. 2013; Bao et al. 2015) was calculated by multiplying the TOC (mg g−1) by mass accumulation rate (MAR; 0.013 g cm−2 y−1). The molar TOC/TN ratio was calculated to infer the origin of sediment organic matter (Meyers and Teranes 2001).
We used the chironomid-inferred mean July temperature from the Lake Atnsjøen core to characterize summer climate conditions (Zawiska et al. 2017). The length of the ice-free season is an additional important climate driver of the ecology in ice-covered lakes. We used the bosminid (Bosminidae) sex ratio from Zawiska et al. (2017) as a proxy for the length of the growing season (Kultti et al. 2011).
Data analysis
A summary of the paleolimnological and historical variables included in the current study is given in ESM Table S1. In this study, species richness and community composition were used as biodiversity proxies to examine temporal changes in the diatom and cladoceran communities. Species richness is one component of biodiversity (Gaston and Spicer 2004) and is simply the number of species present in a sample (Gaston and Spicer 2004). Species composition (relative abundance) was chosen because it is more informative than “species-neutral” diversity indices. Assignment of samples to diatom and cladoceran zones was done using unweighted pair-group average (UPGMA) cluster analysis with a with Bray–Curtis distance as the measure of dissimilarity.
The differential influences of climate and settlement/nutrients on organism communities or productivity were based predominantly on correlation analyses and comparison of the graphs of individual variables. Temporal changes in community composition of diatoms and cladocerans, however, were analysed by principal component analysis (PCA) using Hellinger-transformed relative occurrences of diatoms and cladocerans as input data in the PCA analysis. Chironomid-inferred July temperature and TOC flux were added passively as environmental variables. For the diatom community, percent pelagic species was added passively to examine if changes in the community reflect shifts in the relative abundances of pelagic and benthic species. Likewise, the percentage of large-bodied cladocerans (Moss et al. 2003) was added passively in the PCA of the cladoceran community to infer changes in the community size structure and composition. Relationships between environmental variables and response variables were assessed by Pearson’s correlation coefficient. Non-normally distributed variables were transformed (log10(X + 1)). Statistical analyses were conducted in SPSS Statistics 24 (IBM 2017), PAST (Hammer et al. 2001) and CANOCO 5.0 (ter Braak and Šmilauer 2012).
Results
Diatom community composition and species richness
We recorded 126 diatom taxa in the studied sediment core. The most common species, Tabellaria flocculosa, constituted on average 54% of the diatom community and occurred in high abundances throughout the period studied (Fig. 3). Tetracyclus glans was also rather common, constituting on average 8.5% of the community. The rest of the species occurred at average relative abundances of < 3%. Cluster analysis identified three distinct diatom zones (Figs. 3 and 4): Diatom zone 1, from 1000 CE to 1580 CE (samples 34–16), Diatom zone 2 from 1580 CE to 1710 CE (samples 15-13) and Diatom zone 3 from 1710 CE to present (samples 12–1).
Stratigraphic diagrams from the Lake Atnsjøen sediment core. Upper panel: Relative abundances of the 21 most common diatoms in the sediment. Cyclotella bodanica, C. radiosa and Aulacoseira lirata (not shown in figure) comprise the pelagic species. The rest are benthic/meroplanktonic. Middle panel: Relative abundances of cladocerans in the sediment. Daphnia longispina and Eubosmina comprise the pelagic taxa and the rest are littoral taxa. Lower panel: Diatom PCA1 and PCA2 scores, diatom species richness (SR), percentage of pelagic diatoms, cladoceran PCA1 and PCA2 scores, cladoceran species richness (SR), percentage of pelagic cladocerans and percentage of large-bodied cladocerans. Periods of low (1000–1750 CE) and high (1750 CE–present) human activity are separated by dashed line. Periods of cooling according to Zawiska et al. (2017) are highlighted by grey shading. In the diatom diagram, zones are indicated by Diat 1, Diat 2 and Diat 3, and are separated by grey lines
PCA of the diatom and cladoceran communities in the sediment of Lake Atnsjøen. Upper left panel: PCA ordination plot of the diatom community in the sediment layers from 1 to 34 cm depth. Upper right panel: PCA ordination plot of diatom species (showing the 25 best fitting species on both axes) with the three major diatom zones delineated. Chironomid-inferred July temperature (Temperature), flux of organic carbon (TOC flux) and percentage of pelagic diatoms (pelagic diatoms) are added as supplementary variables and do not affect the PCA analysis. Diatom species in plot are abbreviated as follows: Achnantes minutissima (Aminut), Aulacoseira lirata (Alirata), Cyclotella bodanica (Cbodan), C. radiosa (Cradio), Cymbella minuta (Cminuta), Denticula tenuis (Dtenuis), Eunotia argus (Earcus), E. minor (Eminor), E. muscicola v. tridentula (Emusciv), E. pectinalis (Epect), E. praerupta (Epraer), Fragilaria brevistriata (Fbrevs), F. virescens (Fvires), Frustulia rhomboides v. amphipleuroides (Frombv), Gomphonema acuminatum (Gacumn), Navicula clementis (Nclem), N. pupula (Npupula), N. scutiformis (Nscutf), Neidium ampliatum (Nampli), N. productum (Nprodc), Synedra parasitica (Sparas), Tabellaria quadriseptata (Tquadr), T. ventricosa (Tventr), Tetracyclus glans (Tglans). Lower left panel: PCA ordination plot of the cladoceran community in the sediment layers from 1 to 34 cm depth. Lower right panel: PCA ordination plot of cladoceran species with the three cladoceran zones delineated (samples 23 and 29 were not grouped with any of the other samples). Temperature, TOC flux and large cladocerans are added as supplementary variables and do not affect the PCA analysis. Cladoceran species in plot abbreviated as: Acroperus harpae (Aharp), Alona affinis (Aaff), A. costata (Acost), A. guttata (Agutt), A. intermedia (Ainter), A. quadrangularis (Aquadr), A. rectangula (Arect), Alonella excisa (Aexci), A. nana (Anan), Alonopsis elongata (Aelong), Camptocercus lilljeborgi (Clill), C. rectirostris (Crect), Chydorus sphaericus (Csphae), Daphnia longispina (Dlong), Eubosmina (Eubosm), Eurycercus lamellatus (Elam), Graptolebris testudinaria (Gtest), Kurzia latissima (Klati), Leydigia leydigi (Lley), Paralona pigra (Ppigr), Pleuroxus laevis (Plaev), P. truncates (Ptrunc), Rhynchotalona falcata (Rfalc)
PCA1diat and PCA2diat had eigenvalues of 0.23 and 0.16 and accounted for 23.4% and 15.9% of the variation in the diatom community, respectively. Development of diatom community composition during the past millennium followed a clear trajectory of the PCAdiat scores of the first two PCA axes, shifting between different communities that correspond to the three diatom zones (Figs. 3 and 4). The community of zone 1 had low scores of both PCA1diat and PCA2diat and was characterized to some extent by species like T. glans, Gomphonema acuminatum, Fragilaria brevistriata, Achnanthes minutissima and Fragilaria virescens. The community of zone 2 had higher PCA1diat scores and lower PCA2diat scores compared to zone 1, mainly as a consequence of lower occurrences of A. minutissima, F. virescens and E. minor, and higher occurrences of C. radiosa and C. bodanica. The most characteristic species of zone 2 were Aulacoseira lirata, C. radiosa, T. quadriseptata and Synedra parasitica. The zone 3 assemblage again displayed lower PCA1diat scores and higher PCA2diat scores. The community changed during zone 3, but overall the most characteristic species were N. scutiformis, N. ampliatum, N. clementis and F. rhomboides v. amphipleuroides, Navicula pupula and Eunotia pectinalis. From 1710 CE to 1850 CE the community was relatively stable and still characterized by C. radiosa, but C. bodanica was also typical in the initial part of zone 3. From 1850 CE, the community changed gradually towards even lower PCA1diat scores and higher PCA2diat scores. Thus, from 1950 CE until present, the community clusters around PCA1diat scores around 0 and PCA2diat scores of 0.75–0.84. The most recent shift in the community within zone 3 was a consequence of decreasing occurrences of C. radiosa, C. bodanica, T. glans and T. quadriseptata and increasing frequencies of species such as N. pupula, E pectinalis, F. rhomboides v. amphipleuroides, Neidium ampliatum, and N. scutiformis further up core.
Supplementary variables, including pelagic diatoms, TOC flux and temperature, account for 57.3% of the variation of the fitted response data along PCA1diat (pseudo-canonical correlation 0.88) and for 29.9% along PCA2diat (pseudo-canonical correlation 0.78). PCA1diat was contrasting benthic and pelagic species, as indicated by the strong correlation between pelagic diatoms and PCA1diat (Figs. 3 and 4, r = 0.87, p < 0.01). The most common pelagic species were C. radiosa and C. bodanica, with A. lirata occasionally a subdominant species, for example from 1600 to 1680 CE (data not shown). Over the whole time period studied, PCA2diat was significantly correlated with TOC flux (Fig. 4, r = 0.75, p < 0.01) and with temperature (Fig. 4, r = 0.55, p < 0.01).
Diatom species richness fluctuated throughout the time period studied (Fig. 3). Minimum species richness was recorded around 1600 CE, associated with the maximum PCA1diat scores and minimum in PCA2diat scores. There was a positive, although weak trend in species richness from this time until the most recent period when species richness peaked (Fig. 3 lower panel).
Cladoceran community composition and species richness
We recorded 23 cladoceran taxa in the sediment of Lake Atnsjøen. The most common was Eubosmina, constituting between 71.8 and 91.3% of the community (Fig. 3). Except for Acroperus harpae in the twelth century, no other taxon constituted > 10%. Most of the species recorded are considered littoral species. PCA1clad and PCA2clad had eigenvalues of 0.20 and 0.14 and accounted for 19.9% and 14.3% of the variation in the cladoceran community, respectively. The cluster analysis of the cladoceran community showed no strong zonation in the sediment core (Fig. 3 lower panel and Fig. 4 lower panels). The size structure of the community fluctuated during the study period, as indicated by the variation in the fraction of large-bodied species (D. longispina and E. lamellatus), but showed no specific trend (Fig. 3). Supplementary variables large-bodied cladocerans, TOC flux and temperature account for 57.3% of the variation of the fitted response data along PCA1clad (pseudo-canonical correlation 0.77) and for 15.2% along PCA2clad (pseudo-canonical correlation 0.47). The fraction of large-bodied species was significantly negatively correlated to PCA1clad (r = −0.69, p < 0.01). Cladoceran species richness fluctuated throughout the studied time period, with no specific trend (Fig. 3). Species richness, however, was significantly positively correlated with the fraction of large-bodied species (r = 0.68, p < 0.01). Overall, relatively high occurrence of large-bodied species was associated with low PCA1clad scores and high species richness, whereas relatively low occurrence of large-bodied species was associated with high PCA1clad scores and low species richness.
Lake productivity: flux of consumers and TOC
Accumulation rates (fluxes) of turbellarians and cladocerans (both pelagic and littoral) were low until the fourteenth century (Fig. 5 ). Throughout the core, pelagic cladoceran flux was 3 to 12 times higher than littoral flux, because of the dominance of pelagic Eubosmina. The two, however, show similar trends, and are hereafter referred to collectively as cladoceran flux. Trichopterans were not recorded during the initial period.
Stratigraphic diagram from Lake Atnsjøen sediment core showing trichopteran flux, turbellarian flux, pelagic cladoceran flux, littoral cladoceran flux, TOC flux (Zawiska et al. 2017), ratio of arboreal pollen (AP) to non-arboreal pollen (NAP), human pollen indicators (HPI, Zawiska et al. 2017), chironomid-inferred July air temperature (Zawiska et al. 2017) and bosminid sex ratio (Zawiska et al. 2017), and TOC/TN ratio (molar). Periods of low (1000–1750 CE) and high (1750 CE–present) human activity are indicated in the diagram. Also, periods of cooling, according to Zawiska et al. (2017), are highlighted
Around 1400 CE there was a minor peak in turbellarian and cladoceran fluxes. For cladocerans this was followed by another peak of corresponding size around 1550 CE. Except for these peaks, fluxes of cladocerans and trichopterans were low until 1850–1880 CE. Turbellarian flux increased slightly between about 1750 and 1850 CE. From 1850 to 1880 CE, fluxes of trichopterans and cladocerans, respectively, increased to the highest levels during the whole study period. Turbellarian flux also showed a pronounced increase to a maximum around 1980 CE. This increase was somewhat delayed relative to the rise in cladoceran and trichopteran fluxes. After 1960 CE there was a decrease in flux for trichopterans and somewhat later, about 1980 CE, a similar decrease for cladocerans and turbellarians.
TOC flux showed substantial variation during the last millennium (Fig. 5). Initially it decreased slightly until 1210 CE, followed by a slight increase until 1390. From that time onward the TOC flux displayed a decreasing trend until it reached the lowest recorded value in 1550 CE. Around 1620 CE there was a peak in the TOC flux, followed by a low value in 1680 CE. Then the TOC flux increased slightly until 1850 CE, followed by an accelerated increase until the greatest maximum was reached during the period 1980–2000 CE, corresponding to a 5.5-fold increase in TOC flux. This noteworthy rise in TOC flux coincided with the steep increase in cladoceran and trichopteran flux, but it came somewhat before the rise in turbellarian flux. From 1990 CE onwards TOC flux decreased again, concurrent with the recent decreases in fluxes of trichopterans, turbellarians and cladocerans.
For the whole millennium, temperature was significantly correlated with fluxes of trichopterans, turbellarians, pelagic and littoral cladocerans and TOC (trichopteran flux: r = 0.49, p < 0.01; turbellarian flux: r = 0.54, p < 0.01; pelagic cladoceran flux: r = 0.60, p < 0.01; littoral cladoceran flux: r = 0.67, p < 0.01; TOC flux: r = 0.51, p < 0.01). Furthermore, fluxes of trichopterans, turbellarians, and pelagic and littoral cladocerans were significantly correlated with TOC flux (trichopteran flux: r = 0.68, p < 0.01; turbellarian flux: r = 0.64, p < 0.01; pelagic cladoceran flux: r = 0.67, p < 0.01; littoral cladoceran flux: r = 0.62, p < 0.01).
The molar TOC/TN ratio of the sediment did not show a trend, but varied between 3 and 12 (mostly < 10, Fig. 5) except for one value < 2 (27 cm, ~ 1220 CE) and one > 18 (14 cm, ~ 1620 CE). On the basis of a relatively high intercept of the TOC versus TN plot, it appears that there is some contribution from inorganic N in the sediments (ESM Fig. S3).
Discussion
Ecological changes 1000–1750 CE
The climate reconstruction showed large variation from 1000 to 1750 CE, as indicated by alternation between colder and warmer phases (Zawiska et al. 2017). During this long period, human activity was relatively low and climate was a strong driver of the Lake Atnsjøen ecosystem properties. The most conspicuous ecological change during this period was related to biodiversity, i.e. changes in the diatom community starting with the transition from the pre-1580 CE community towards the 1580–1710 CE community (Figs. 3 and 4). This change coincided with a considerable drop and subsequent increase in diatom species richness. The change spanned the transition from a warmer to a colder climate during the LIA. The community change involved a shift towards pelagic species dominance; in particular, C. radioasa increased, but C. bodanica and A. lirata also became more common. During this phase, climate changed from having relatively warm and short summers (relatively high July temperatures and high bosminid sex ratios), towards longer and colder summers (lower July temperature and lower bosminid sex ratios). The increase in pelagic species at this time could have been caused by stronger mixing of the water column during the long, cold summers, caused by ocean-dominated climate that favored relatively large pelagic species (Dressler et al. 2011). Aulacoseira, especially, has been associated with increased periods of turbulence (Rühland et al. 2015). In general, smaller cyclotelloid species like D. stelligera and C. commensis seem to increase during periods of stronger water column stratification (Rühland et al. 2015). The Cyclotella species that occur in Lake Atnsjøen, C. bodanica and C. radiosa, are both relatively large species. Rühland et al. (2015) also suggested that larger cyclotelloid species may increase during periods of more turbulence in the water column. The shift between benthic and pelagic species could also be related to development of the littoral zone. Whereas low occurrences of quillwort (Isöetes lacustris) are associated with both high and low frequencies of pelagic species in Lake Atnsjøen, higher frequencies of this macrophyte were only accompanied by lower frequencies of pelagic diatoms (data not shown). Length of the growing season may have a strong impact on macrophyte communities (Lacoul and Freedman 2006). Varying growing season length could have impacted the abundance of I. lacustris through effects on light and temperature and/or the availability of nutrients (via stratification). Higher occurrences of I. lacustris, reflecting development of the littoral zone, would favor benthic diatom species and vice versa. The transition from the 1580–1710 CE diatom community to the 1710–1850 CE community is discussed further below.
Climate shifts also influenced lake productivity until 1750 CE, as indicated by changes in fluxes of consumers and TOC (Manca et al. 2007). The first cold period, 1050–1150 CE, was associated with a slight decrease in fluxes of TOC and turbellarians. A very low TOC/TN ratio occurred during the warm period from 1150 to 1270 CE, but it was not accompanied by increased TOC flux. Presence of inorganic N in the sediment could explain the low TOC/TN ratio. Inorganic nitrogen in the sediment could have several sources. Nitrogen released from decomposition of organic matter within the sediments has little impact on the TOC/TN ratio, so the low ratio could be a consequence of inorganic nitrogen from catchment soils, e.g. as ammonia adsorbed to clay particles (Schubert and Calvert 2001), or from volcanic eruptions (Robinson 1994). We cannot, however, identify the nitrogen sources using our data.
The warmer period from 1370 CE to 1420 CE was associated with increasing fluxes of TOC, cladocerans and turbellarians. A short, very cold period in the 15th century affected lake productivity negatively, as indicated by decreasing fluxes of TOC, cladocerans and turbellarians. The subsequent short, moderately warm period from 1490 to 1550 CE led to minor increases in TOC and cladoceran fluxes. A longer cold phase from 1550 to 1800 CE, the LIA, ended the period of lower human activity and overlapped with the period of human settlement that began after 1750 CE (Table 1). This long cold period had a strong impact on lake productivity, as was observed at other lakes in Scandinavia (Luoto et al. 2008). In Lake Atnsjøen, it manifested as decreased fluxes of TOC and consumers. The peak in TOC/TN ratio around 1620 CE suggests that soil-derived organic matter from the catchment may have contributed to the coincident local peak in TOC flux (Meyers 1994). In addition to low summer temperatures, climate impacts during the LIA also seemed to be mediated by a shorter open-water season, as indicated by the increasing bosminid sex ratio.
Ecological changes: 1750 CE to present
The most striking ecological change during the last 250 years of increasing human activity was related to changes in lake productivity. The initial part of this period overlapped with the LIA, as noted above, associated with dramatic climate changes. Initially, the length of the growing season increased (decreasing bosminid sex ratio) and the time window from 1750 CE to 1880 CE was associated with an unprecedented increase in temperature (4.4 °C). During the first part of the settlement period there were no major changes in fluxes of TOC and consumers. From 1850 to 1900 CE productivity began to increase, as indicated by increasing fluxes of TOC, trichopterans, turbellarians and cladocerans. Hence, the pronounced temperature increases caused only a moderate increase in fluxes. Low temperatures may have damped potential productivity effects of the initial human settlement in this time window.
The fluxes of TOC, trichopterans, turbellarians and cladocerans, all showed a remarkable increase after 1880 CE, peaking in the period 1950–1980 CE, although the exact timing differed slightly among proxies. This was after the period of accelerated climate warming. From 1880 CE to present, July temperature showed only a minor increase and low variation. Hence, climate was of minor importance for this increase in lake productivity. The increase in productivity paralleled the increase in human settlements, suggesting that greater human activity contributed to the rise in lake productivity. Additionally, several changes in land use in the catchment occurred in this period. This involved changes in agriculture and industrialization (Table 1 and references cited therein). Forestry was mechanized ca. 1950 CE and in subsequent decades. Thus, there was land-use intensification in the catchment, which stimulated lake productivity even after the number of settlements and the population size decreased from 1950 CE. Although the area around Lake Atnsjøen was not used for crop cultivation because of harsh climate and nutrient-poor soils (Brænd 1989), extensive agricultural practices, including grazing and hay production (Table 1), may have contributed to increased erosion and nutrient flux from the catchment (Massa et al. 2012). Likewise, deforestation during the settlement period (Table 1) likely also contributed to increased erosion and washout of nutrients (Koinig et al. 2003). Intensification of land use, beginning about 1920 CE, would have amplified this process. For example, cultivation of infields/pastures and initiation and increasing use of chemical fertilizers between the world wars, in addition to the mechanization of forestry, likely also contributed to greater input of nutrients to the catchment, thereby stimulating lake productivity (Bragée et al. 2013; Millet et al. 2014). Even though increasing human activity in the catchment of Lake Atnsjøen stimulated lake productivity, it did not manifest in decreased TOC/TN ratios. On the contrary, the TOC/TN ratio increased somewhat from 1880 CE onwards. Likewise, a study by Millet et al. (2014) at Lake Igaliku in southern Greenland showed that extensive Norse farming (grazing of cattle, sheep and goats) from 1000 to 1400 CE, as well as Norse-analog farming from 1900 to 1980 CE, elicited increased TOC flux and higher TOC/TN ratio in the sediments. It was only with the shift from Norse-analog farming to modern practices after 1980 that the sediment TOC/TN ratio decreased, showing a strong human impact on the lake ecosystem. Apparently, anthropogenic pressures in the Lake Atnsjøen catchment, even during the periods of highest human activity, were too low to elicit a decrease in sediment TOC/TN ratio.
Low molar TOC/TN ratio of the sediment (< 10) indicates that the carbon pool in the sediment was mostly autochthonous in origin (Meyers 1994). Lake Atnsjøen is relatively large and deep, with a very poorly developed littoral zone because of steep slopes close to the lake shore. The lake has a sparse macrophyte community, consisting mainly of I. lacustris (Halvorsen 2004; Zawiska et al. 2017). The contribution of littoral primary producers to total primary production in large and deep lakes is relatively small (Doi 2009). Hence, phytoplankton production likely dominated primary production in Lake Atnsjøen, and is confirmed by the low TOC/TN values.
Decreasing fluxes of TOC, trichopterans, turbellarians and cladocerans, during the most recent decades, indicate decreasing lake productivity, which is supported by lake monitoring data. In 1980 CE average concentrations of phytoplankton biomass, total phosphorus and total nitrogen were 240 mm3/m3, 7.4 µg l−1 and 396 µg l−1, respectively (Holtan et al. 1982). For the period 2003–2011 CE these variables had decreased to 132 mm3/m3, 4.2 µg l−1 (2005–2011 CE) and 135 µg l−1 (2005–2009 CE, T.C. Jensen unpublished data), respectively, indicating decreased lake productivity. Nevertheless, the lake was oligotrophic even during the most productive period. Decreased lake productivity after 1980 CE corresponds with information about human settlement/activity and land use in the area. From 1950 CE, the number of permanent settlements with agricultural activity (livestock and/or land used for fodder production) in the catchment decreased by 25%, and in the neighboring Folldal municipality the population decreased by 27% from 1950 CE to present (Table 1, Dahl [1960]; A. Alander pers. commun.; H.E. Nesset pers. commun.). Apparently, this overall reduction in human pressure resulted in decreased lake productivity, likely a consequence of reduced erosion and input of nutrients to the lake from the catchment. Overall, both climate and human presence/activity affected productivity in Lake Atnsjøen during the settlement period, but productivity was more closely related to human presence and changes in land use than to climate, especially during the last 130 years.
The transition from the 1580–1710 CE diatom community to the 1710–1850 CE community coincided with the end of the LIA and the beginning of accelerated climate warming, with strong impact on the length of the growing season. The 1710–1850 CE community also overlapped with the initial settlement period. Low TOC fluxes at that time, however, indicated that human impact from 1800 to 1850 CE was still very low. Hence, climate was probably still a major driver of the diatom community, explaining the decreasing frequencies of pelagic A. lirata and pelagic/benthic T. quadriseptata (Rühland et al. 2015). The change in the diatom community from 1850 CE to the post-1950 community was also associated with a decrease in pelagic species, initially C. radiosa, and later, C. bodanica. The almost complete disappearance of pelagic species after 1960 CE is probably partly climate-induced, caused by warm, relatively long summers with stronger stratification of the water column. As the increase in lake productivity in Lake Atnsjøen after 1850 CE was relatively moderate, within the ultraoligotrophic to oligotrophic range, one would not expect a major shift in diatom species composition. In accordance with this, most of the diatom species recorded are oligotrophic or oligotrophic/mesotrophic indicators (Philibert and Prairie 2002; Miettinen 2003; Bigler et al. 2007; Chen et al. 2008; Ptacnik et al. 2008; Hobæk et al. 2012). Some of the species, however, that appeared in higher frequencies after 1850 CE are mesotrophic (N. pupula, N. ampliatum) or even eutrophic indicators (N. clemensis). It thus appears that increasing human settlement and activity after 1850 CE, in addition to climate, had a moderate impact on the diatom community, which is indicative of a slightly higher trophic status than before 1850 CE.
Effects of human impacts on productivity and biodiversity
Bottom-up control of aquatic food webs is typically strongest at the base of the food web and decreases at higher trophic levels (McQueen et al. 1986). Our results from Lake Atnsjøen agree with this claim. The magnitude of the bottom-up signal, however, seemed to differ for biodiversity and lake productivity. Regarding biodiversity, the increase in human activity was associated with a moderate change in diatom community composition, i.e. relative abundances, but not species richness. This bottom-up influence on biodiversity, however, did not propagate up the food web to the consumers, as no noticeable effect on cladoceran community composition and species richness was detected. In contrast, the bottom-up signal mediated by human impact, resulting in increased TOC flux, cascaded up the food web, resulting in greater fluxes of different groups of consumers (trichopterans, turbellarians and cladocerans).
There are several possible reasons for different responses to increasing human activity around Lake Atnsjøen with respect to biodiversity and lake productivity. Factors other than human impact may have been more important in regulating biodiversity in Lake Atnsjøen. For example, silicon is an important nutrient for diatoms and has been shown to be an important driver of diatom communities (Willén 1991). The long-term decrease of T. glans in Lake Atnsjøen from 1500 CE onwards, might be related to silica availability, as this species has relatively high Si requirements (Michel et al. 2006). Regarding consumers, the cladoceran community in Lake Atnsjøen could be under stronger top-down, relative to bottom-up control. We observed a change in the size ratio of the cladoceran community with the change in community composition and species richness. Fish predation often has an overriding impact on this zooplankton community trait, with large-bodied species favored at relative low levels of fish predation, and smaller species favored under conditions of higher fish predation (Brooks and Dodson 1965). The most common fish species in Lake Atnsjøen are Arctic char (Salvelinus alpinus) and brown trout (Salmo trutta), which dominate the pelagic and littoral zones, respectively. Although both species utilize crustacean zooplankton (largely cladocerans) as food, char is the most important zooplankton predator in Lake Atnsjøen and shows a strong preference for larger species like Daphnia (Saksgård and Hesthagen 2004, O.T. Sandlund et al. unpublished data). We suggest that changing fish predation was a main driver of cladoceran diversity (community composition and species richness) in Lake Atnsjøen over the last millennium. Furthermore, we cannot exclude the possibility that zooplankton exert a top-down control on the diatom assemblages in Lake Atnsjøen through grazing, although losses of diatoms to zooplankton grazing are likely more important at higher trophic state (Sommer et al. 1986). Finally, the different responses to increasing human activity, with respect to biodiversity and productivity in Lake Atnsjøen, could relate to the range of changes in trophic state/nutrient concentrations that Lake Atnsjøen has endured. The lake constitutes a low-resource environment with low concentrations of nutrients and low phytoplankton biomass. Even during the most productive period in the lake, from 1960 to 1990 CE, the lake was still oligotrophic (Holtan et al. 1982). The cladoceran community in the Lake Atnsjøen sediment resembles that typically encountered in nutrient-poor Norwegian lakes, characterized by oligotrophic indicators. For example, the dominant cladoceran taxon throughout the millennium, Eubosmina, is a common species that often dominates the zooplankton in ultraoligotrophic to oligotrophic lakes. In more productive lakes it is replaced by B. longirostris (Jensen et al. 2013). Thus, from the limited change in productivity experienced in Lake Atnsjøen, one would not expect a major species replacement (Jensen et al. 2013), in agreement with the low species turnover in Lake Atnsjøen (short primary DCA-axes cladocerans, 0.99). For comparison, a slight increase in resource availability in the low-resource range for cladocerans causes a major increase in cladoceran growth/production (Urabe 1991; Lampert and Trubetskova 1996). In ultraoligotrophic and oligotrophic lakes, a minor increase in nutrient input and primary productivity may therefore lead to significant bottom-up cascades on productivity of consumers because consumers are somewhat relieved from strong resource limitation.
There are few comprehensive single-lake studies of increasing human impact/nutrient loading in oligotrophic lakes that include aspects of both biodiversity and productivity and consider multiple trophic levels. Oligotrophic, perialpine Lake Annecy, was studied intensively and showed a period of human-induced eutrophication ca. 1950-1980, followed by oligotrophication (Perga et al. 2010; Alric et al. 2013; Berthon et al. 2014). In contrast to Lake Atnsjøen, both the productivity signal and the biodiversity signal in Lake Annecy propagated to the trophic level of the consumers, in response to increasing nutrient loading. Differences in human pressure and different ranges of change in trophic state likely explain the different ecological responses of the two lakes. Lake Atnsjøen, exposed to low human pressures, underwent change in the ultraoligotrophic to oligotrophic range. Lake Annecy, exposed to much greater human pressure (Crook et al. 2004), experienced a change in the oligotrophic-mesotrophic range (Perga et al. 2010; Berthon et al. 2014). Hence, the trophic state in Lake Annecy was skewed toward optima for mesotrophic and eutrophic indicators during the more productive period. For example, the eutrophic indicator B. longirostris appeared in the lake after eutrophication (Perga et al. 2010).
Conclusions
Our study of Lake Atnsjøen showed that even low levels of human activity can have profound effects on oligotrophic subalpine lake ecosystems. Climate was the primary driver of lake ecology in the first 750 years of the study period, whereas human presence and changes in land use were more important drivers of ecosystem change in the last two centuries, especially during the most recent 130 years. Our study emphasizes the need to study human and climate impacts on aquatic ecosystems in concert. The results also demonstrate that increasing trophic status in the ultraoligotrophic to oligotrophic range may have different effects on productivity and biodiversity, which has implications for monitoring and management of freshwaters. Many biological indices used for assessment of water quality are based on changes in the diversity of biological communities in response to a given stressor, for example eutrophication. Because increasing nutrient loading of lakes at the very low end of the trophic state spectrum may have stronger effects on production than on biodiversity, the indices based on biological diversity may not capture this early evidence of eutrophication. This study highlights the importance of emphasizing aspects of productivity in biological indices that are used to assess water quality, thus providing an early warning signal for the eutrophication process.
References
Alric B, Jenny J-P, Berthon V, Arnaud F, Pignol C, Reyss J-L, Sabatier P, Perga M-E (2013) Local forcings affect lake zooplankton vulnerability and response to climate warming. Ecology 94:2767–2780
Anderson NJ, Renberg I, Segerstrom U (1995) Diatom production responses to the development of early agriculture in a boreal forest lake-catchment (Kassjon, Northern Sweden). J Ecol 1995:809–822
Bækkelund B, Kalvatn SJ (1998) Bygdebok for Folldal bind VI. Folldal verks historie ca 1745–1993. [Local history book for Folldal volume VI. The history of Folldal mine approximately 1745–1993.]. Folldal bygdeboknemnd, Folldal, p 308
Bao R, Hernandez A, Saez A, Giralt S, Prego R, Pueyo JJ, Moreno A, Valero-Garces BL (2015) Climatic and lacustrine morphometric controls of diatom paleoproductivity in a tropical Andean lake. Quat Sci Rev 129:96–110
Battarbee RW (1986) Diatom analysis. In: Berglund BE (ed) Handbook of Holocene palaeoecology and palaeohydrology. Wiley, Chichester, pp 527–570
Berthon V, Alric B, Rimet F, Perga ME (2014) Sensitivity and responses of diatoms to climate warming in lakes heavily influenced by humans. Freshw Biol 59:1755–1767
Bigler C, von Gunten L, Lotter AF, Hausmann S, Blass A, Ohlendorf C, Sturm M (2007) Quantifying human-induced eutrophication in Swiss mountain lakes since AD 1800 using diatoms. Holocene 17:1141–1154
Birks HH (2007) Plant macrofossil introduction. In: Elias SA (ed) Encyclopedia of quaternary science. Elsevier, Amsterdam, pp 2266–2288
Brænd B (1989) Sollia 1. Eldre generell historie, garder og slekter i Øverdalen skolekrets [Sollia 1. Older general history, farms and families in Øverdalen school district]. Stor-Elvdal kommune, Koppang, p 485
Brænd B (2007) Sollia 2. Generell historie 1862–1945, garder i statsallmenningen og slekter fra Setningssjøen til Holtet i Atnbrua skolekrets [Sollia 2. General history 1862–1945, farms in the state community and families from Lake Setningssjøen to Holtet in Atnbrua school district]. Stor-Elvdal kommune, Koppang, p 414
Brænd B (2009) Sollia 3. Generell historie etter 1945, garder og slekter i Atnelien i Atnbrua skolekrets [Sollia 3. General history after 1945, farms and families in Atnelien in Atnbrua school district]. Stor-Elvdal kommune, Koppang, p 383
Bragée P, Choudhary P, Routh J, Boyle JF, Hammarlund D (2013) Lake ecosystem responses to catchment disturbance and airborne pollution: an 800-year perspective in southern Sweden. J Paleolim 50:545–560
Brooks JL, Dodson SI (1965) Predation, body size, and composition of plankton. Science 150:28–35
Callicott JB, Crowder LB, Mumford K (1999) Current normative concepts in conservation. Conserv Biol 13:22–35
Cao YM, Zhang EL, Langdon PG, Liu EF, Shen J (2014) Chironomid-inferred environmental change over the past 1400 years in the shallow, eutrophic Taibai Lake (south-east China): Separating impacts of climate and human activity. Holocene 24:581–590
Chen GJ, Dalton C, Leira M, Taylor D (2008) Diatom-based total phosphorus (TP) and pH transfer functions for the Irish Ecoregion. J Paleolimnol 40:143–163
Clark BJ, Paterson AM, Jeziorski A, Kelsey S (2010) Assessing variability in total phosphorus measurements in Ontario lakes. Lake Reservoir Manag 26:63–72
Colby P, Spangler G, Hurley D, McCombie A (1972) Effects of eutrophication on salmonid communities in oligotrophic lakes. J Fish Res Board Can 29:975–983
Crook D, Siddle D, Dearing J, Thompson R (2004) Human impact on the environment in the Annecy Petit Lac catchment, Haute-Savoie: a documentary approach. Env Hist 10:247–284
Dahl BP (1960) Ad Novas. Utviklingen av bosetning, eiendoms- og bruksforhold i Nordre Atnadalen [Development of settlement, property and use conditions in Nordre Atnadalen]. Universitetsforlaget, Oslo–Bergen, pp 1–145
Davidson TA, Jeppesen E (2013) The role of palaeolimnology in assessing eutrophication and its impact on lakes. J Paleolimnol 49:391–410
Dodson SI, Arnott SE, Cottingham KL (2000) The relationship in lake communities between primary productivity and species richness. Ecology 81:2662–2679
Doi H (2009) Spatial patterns of autochthonous and allochthonous resources in aquatic food webs. Popul Ecol 51:57-64
Dong X, Bennion H, Battarbee RW, Sayer CD (2012a) A multiproxy palaeolimnological study of climate and nutrient impacts on Esthwaite Water, England over the past 1200 years. Holocene 22:107–118
Dong X, Bennion H, Maberly SC, Sayer CD, Simpson GL, Battarbee RW (2012b) Nutrients exert a stronger control than climate on recent diatom communities in Esthwaite Water: evidence from monitoring and palaeolimnological records. Freshw Biol 57:2044–2056
Dressler M, Schwarz A, Hubener T, Adler S, Scharf BW (2011) Use of sedimentary diatoms from multiple lakes to distinguish between past changes in trophic state and climate: evidence for climate change in northern Germany during the past 5000 years. J Paleolimnol 45:223–241
Dudgeon D, Arthington AH, Gessner MO, Kawabata Z-I, Knowler DJ, Lévêque C, Naiman RJ, Prieur-Richard A-H, Soto D, Stiassny ML (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev Camb Philos Soc 81:163–182
Frey DG (1986) Cladocera analysis. In: Berglund BE (ed) Handbook of Holocene palaeoecology and palaeohydrology. Wiley, Chichester, pp 667–692
Gaston KJ, Spicer JI (2004) Biodiversity: an introduction. Blackwell Publ, Malden
Goldewijk KK (2005) Three centuries of global population growth: a spatial referenced population (density) database for 1700–2000. Pop Env 26:343–367
Goldewijk KK, Beusen A, Janssen P (2010) Long-term dynamic modeling of global population and built-up area in a spatially explicit way: HYDE 3.1. Holocene 20:565–573
Halvorsen G (2004) Some physical and chemical characteristics of Lake Atnsjøen. Hydrobiologia 521:129–140
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9
Hessen DO, Faafeng BA, Andersen T (1995) Replacement of herbivore zooplankton species along gradients of ecosystem productivity and fish predation pressure. Can J Fish Aquat Sci 52:733–742
Hobæk A, Løvik JE, Röhrlack T, Moe SJ, Grung M, Bennion H, Clarke G, Piliposyan GT (2012) Eutrophication, recovery and temperature in Lake Mjøsa: detecting trends with monitoring data and sediment records. Freshw Biol 57:1998–2014
Holtan H, Brettum P, hals B, Holtan G (1982) Glåma i Hedmark. Delrapport om innsjøer. Undersøkelser i tidsrommet 1978–1980 [The River Glåma in Hedmark. Interim report on lakes. Surveys during the period 1978–1980]. NIVA-rapport. NNIVA, Oslo, p 96
IBM (2017) IBM SPSS statistics for windows. IBM Corp, Armonk
IPCC (2013) IPCC (2013) Summary for Policymakers. In: Stocker TFQD, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013: the physical science basis contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, p 27
Jensen TC, Dimante-Deimantovica I, Schartau AK, Walseng B (2013) Cladocerans respond to differences in trophic state in deeper nutrient poor lakes from Southern Norway. Hydrobiologia 715:101–112
Jeppesen E, Peder Jensen J, Søndergaard M, Lauridsen T, Landkildehus F (2000) Trophic structure, species richness and biodiversity in Danish lakes: changes along a phosphorus gradient. Freshw Biol 45:201–218
Katz NJ, Katz SV, Skobiejeva EI (1977) Atlas rastitielnych ostatkov v torfach. Nedra, Moskva (in Russian)
Koinig KA, Shotyk W, Lotter AF, Ohlendorf C, Sturm M (2003) 9000 years of geochemical evolution of lithogenic major and trace elements in the sediment of an alpine lake–the role of climate, vegetation, and land-use history. J Paleolimnol 30:307–320
Korhola A, Sorvari S, Rautio M, Appleby P, Dearing J, Hu Y, Rose N, Lami A, Camerson N (2002) A multi-proxy analysis of climate impacts on the recent development of subarctic Lake Saanajärvi in Finnish Lapland. J Paleolimnol 28:59–77
Krammer K, Lange-Bertalot H (1986) Bacillariophyceae. 1. Teil: Naviculaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa. Gustav FischerVerlag, Stuttgart
Krammer K, Lange-Bertalot H (1988) Bacillariophyceae. 2. Teil: Bacillariaceae Epithemiaceae, Surirellaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart
Krammer K, Lange-Bertalot H (1991a) Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart
Krammer K, Lange-Bertalot H (1991b) Bacillariophyceae. 4. Teil: Achnanthaceae, Kritische-Erganzungen zu Navicula (Lineolatae) und Gomphonema, Gesamtliteraturverzeichnis. In: Ettl H, Gartner G, Gerlof J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart
Kultti S, Nevalainen L, Luoto TP, Sarmaja-Korjonen K (2011) Subfossil chydorid (Cladocera, Chydoridae) ephippia as paleoenvironmental proxies: evidence from boreal and subarctic lakes in Finland. Hydrobiologia 676:23
Kurek J, Korosi JB, Jeziorski A, Smol JP (2010) Establishing reliable minimum count sizes for cladoceran subfossils sampled from lake sediments. J Paleolimnol 44:603–612
Lacoul P, Freedman B (2006) Relationships between aquatic plants and environmental factors along a steep Himalayan altitudinal gradient. Aquat Bot 84:3–16
Lampert W, Trubetskova I (1996) Juvenile growth rate as a measure of fitness in Daphnia. Funct Ecol 10:631–635
Luoto TP, Nevalainen L, Sarmaja-Korjonen K (2008) Multiproxy evidence for the ‘Little Ice Age’ from Lake Hamptrask, southern Finland. J Paleolimnol 40:1097–1113
Manca M, Torretta B, Comoli P, Amsinck SL, Jeppesen E (2007) Major changes in trophic dynamics in large, deep sub-alpine Lake Maggiore from 1940s to 2002: a high resolution comparative palaeo-neolimnological study. Freshw Biol 52:2256–2269
Massa C, Bichet V, Gauthier É, Perren BB, Mathieu O, Petit C, Monna F, Giraudeau J, Losno R, Richard H (2012) A 2500 year record of natural and anthropogenic soil erosion in South Greenland. Quat Sci Rev 32:119–130
McGowan S, Barker P, Haworth EY, Leavitt PR, Maberly SC, Pates J (2012) Humans and climate as drivers of algal community change in Windermere since 1850. Freshw Biol 57:260–277
McQueen DJ, Post JR, Mills EL (1986) Trophic relationships in freshwater pelagic ecosystems. Can J Fish Aquat Sci 43:1571–1581
Meyers PA (1994) Preservation of elemental and isotopic source identification of sedimentary organic-matter. Chem Geol 114:289–302
Meyers PA, Teranes JL (2001) Sediment Organic Matter. In: Last WM, Smol JP (eds) Tracking environmental change using lake sediments: physical and geochemical methods. Springer, Dordrecht, pp 239–269
Michel TJ, Saros JE, Interlandi SJ, Wolfe AP (2006) Resource requirements of four freshwater diatom taxa determined by in situ growth bioassays using natural populations from alpine lakes. Hydrobiologia 568:235–243
Miettinen JO (2003) A diatom-total phosphorus transfer function for freshwater lakes in southeastern Finland, including cross-validation with independent test lakes. Boreal Environ Res 8:215–228
Mikkelsen E (1980) Varia. Verneplan for vassdrag 10 års vernede vassdrag Kulturminner i Atnavassdraget, Hedmark–Oppland [Protection plan for watercourse 10 years of protected watercourse Cultural heritage in the Atna watercourse, Hedmark–Oppland]. Universitetet i Oslo. Universitetets oldsaksamling, Oslo, pp 1–126
Millet L, Massa C, Bichet V, Frossard V, Belle S, Gauthier E (2014) Anthropogenic versus climatic control in a high-resolution 1500-year chironomid stratigraphy from a southwestern Greenland lake. Quat Res 81:193–202
Moss B, Stephen D, Alvarez C, Becares E, Van de Bund W, Collings SE, Van Donk E, De Eyto E, Feldmann T, Fernandez-Alaez C, Fernandez-Alaez M, Franken RJM, Garcia-Criado F, Gross EM, Gyllstrom M, Hansson LA, Irvine K, Jarvalt A, Jensen JP, Jeppesen E, Kairesalo T, Kornijow R, Krause T, Kunnap H, Laas A, Lille E, Lorens B, Luup H, Miracle MR, Noges P, Noges T, Nykanen M, Ott I, Peczula W, Peeters E, Phillips G, Romo S, Russell V, Salujoe J, Scheffer M, Siewertsen K, Smal H, Tesch C, Timm H, Tuvikene L, Tonno I, Virro T, Vicente E, Wilson D (2003) The determination of ecological status in shallow lakes—a tested system (ECOFRAME) for implementation of the European Water Framework Directive. Aquat Conserv 13:507–549
Neil K, Gajewski K (2017) Impacts of late-Holocene climate variability and watershed-lake interactions on diatom communities in Lac Brule, Quebec. Ecosphere 8:17
Nordli PO, Grimenes AA (2004) The climate of Atndalen. Hydrobiologia 521:7–20
Oeding P (1990) The black death in Norway. Tidsskr Nor Lægef 30:2204–2208
Perga M-E, Desmet M, Enters D, Reyss J-L (2010) A century of bottom-up-and top-down driven changes on a lake planktonic food web: a paleoecological and paleoisotopic study of Lake Annecy, France. Limnol Oceanogr 55:803–816
Perren BB, Massa C, Bichet V, Gauthier É, Mathieu O, Petit C, Richard H (2012) A paleoecological perspective on 1450 years of human impacts from a lake in southern Greenland. Holocene 22:1025–1034
Philibert A, Prairie YT (2002) Diatom-based transfer functions for western Quebec lakes (Abitibi and Haute Mauricie): the possible role of epilimnetic CO2 concentration in influencing diatom assemblages. J Paleolimnol 27:465–480
Ptacnik R, Lepisto L, Willen E, Brettum P, Andersen T, Rekolainen S, Solheim AL, Carvalho L (2008) Quantitative responses of lake phytoplankton to eutrophication in Northern Europe. Aquat Ecol 42:227–236
Robinson C (1994) Lago Grande di Monticchio, southern Italy: a long record of environmental change illustrated by sediment geochemistry. Chem Geol 118:235–254
Routh J, Choudhary P, Meyers PA, Kumar B (2009) A sediment record of recent nutrient loading and trophic state change in Lake Norrviken, Sweden. J Paleolimnol 42:325–341
Rühland KM, Paterson AM, Smol JP (2015) Lake diatom responses to warming: reviewing the evidence. J Paleolimnol 54:1–35
Saksgård R, Hesthagen T (2004) A 14-year study of habitat use and diet of brown trout (Salmo trutta) and Arctic charr (Salvelinus alpinus) in Lake Atnsjøen, a subalpine Norwegian lake. Hydrobiologia 521:187–199
Schrader H, Gersonde R (1978) Diatoms and silicoflagellates. In: Zachariasse et al. (ed) Microplaeontological counting methods and techniques-an excercise on an eight metres section of the lower Pliocene of Capo Rossello Sicily, Utrecht micropaleontological bulletins. Utrecht University, Utrecht, pp 129–176
Schubert CJ, Calvert S (2001) Nitrogen and carbon isotopic composition of marine and terrestrial organic matter in Arctic Ocean sediments: implications for nutrient utilization and organic matter composition. Deep Sea Res Part I 48:789–810
Sommer U, Gliwicz ZM, Lampert W, Duncan A (1986) The PEG-model of seasonal succession of planktonic events in fresh waters. Arch Hydrobiol 106:433–471
Statistics Norway (2018) Temakart for befolkning. [Population maps]
Szeroczyfiska K, Sarmaja-Korjonen K (2007) Atlas of subfossil Cladocera from central and northern Europe. Friends of the lower Vistula Society, Świecie
ter Braak CJF, Šmilauer P (2012) CANOCO Reference manual and user’s guide: software for ordination (version 5.0). Microcomputer power, Ithaca
Tylmann W, Bonk A, Goslar T, Wulf S, Grosjean M (2016) Calibrating 210Pb dating results with varve chronology and independent chronostratigraphic markers: problems and implications. Quat Geochr 32:1–10
Urabe J (1991) Effect of food concentration on growth, reproduction and survivorship of Bosmina–Longirostris (cladocera)—an experimental-study. Freshw Biol 25:1–8
Velichkevich FU, Zastawniak E (2008) Atlas of the Pleistocene vascular plant macrofossils of Central and Eastern Europe. Part 2—Herbaceous dicotyledons. Polish Academy of Science, Kraków
Wang L, Mackay AW, Leng MJ, Rioual P, Panizzo VN, Lu HY, Gu ZY, Chu GQ, Han JT, Kendrick CP (2013) Influence of the ratio of planktonic to benthic diatoms on lacustrine organic matter delta C-13 from Erlongwan maar lake, northeast China. Org Geochem 54:62–68
Whitehead P, Wilby R, Battarbee R, Kernan M, Wade AJ (2009) A review of the potential impacts of climate change on surface water quality. Hydro Sci J 54:101–123
Willén E (1991) Planktonic diatoms-an ecological review. Algo Stud 62:69–106
Wolfe AP, Hobbs WO, Birks HH, Briner JP, Holmgren SU, Ingólfsson Ó, Kaushal SS, Miller GH, Pagani M, Saros JE (2013) Stratigraphic expressions of the Holocene–Anthropocene transition revealed in sediments from remote lakes. Earth Sci Rev 116:17–34
Zawiska I, Luoto TP, Nevalainen L, Tylmann W, Jensen TC, Obremska M, Slowinski M, Woszczyk M, Schartau AK, Walseng B (2017) Climate variability and lake ecosystem responses in western Scandinavia (Norway) during the last Millennium. Paleogeogr Paleoclimatol Paleoecol 466:231–239
Acknowledgements
This research was funded by the EEA and Norway Grants (Grant No. FSS/2013/IIC/W/0022) and the Norwegian Institute for Nature Research. We thank H.E. Nesset, A. Alander and B. Brænd for help and information about the local history of the study area. We thank Kristin Thorsrud Teien for linguistic corrections. The manuscript also benefited from valuable suggestions and comments of two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jensen, T.C., Zawiska, I., Oksman, M. et al. Historical human impact on productivity and biodiversity in a subalpine oligotrophic lake in Scandinavia. J Paleolimnol 63, 1–20 (2020). https://doi.org/10.1007/s10933-019-00100-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-019-00100-5