Abstract
Chydorids (Cladocera, Chydoridae) have two reproductive strategies: asexual reproduction that prevails during favorable environmental conditions and sexual reproduction that is induced by environmental stimuli associated with seasonal or aperiodic environmental stresses. These modes of reproduction can be recognized in the subfossil sedimentary records as parthenogenetic shells of females (asexual reproduction) and by ephippia (sexual reproduction). We studied the interrelations between subfossil chydorid ephippia and environmental variables by analyzing surface sediment samples obtained from 76 Finnish lakes across a latitudinal gradient (60–70°N). The results showed that the total chydorid ephippia (TCE) increases along the climate gradient from ~2 to 3% in the south to ~25% in the north and suggested a significant dependence (r ~ −0.8, P < 0.001) with several climate factors, especially that of mean July air temperature. We used this relationship to create a model for reconstructing past mean July air temperatures. A linear regression of the log10 transformed TCE as a single independent variable explained 76% (SE ± 0.76°C) of the variance of the observed mean July air temperatures. Accordingly, we propose that this novel tool may be highly suitable for reconstructing paleotemperatures in cold-temperate environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In northern boreal and subarctic lakes, the reproduction of littoral water fleas of the family Chydoridae (chydorids) occurs during either asexual (parthenogenetic) or sexual (gamogenetic) reproductive periods. Most of the open-water season, i.e., when the lake is free from ice-cover, asexual reproduction prevails and it is used as a mechanism for rapid population growth and maintenance. Sexual reproduction takes place as a survival strategy when a seasonal (e.g., decreasing water temperature, shortening of the photoperiod) or aperiodic environmental stress triggers the change in reproduction (Kiser et al., 1963; Frey, 1982; Nevalainen & Sarmaja-Korjonen, 2008a, b). In chydorid populations, sexual reproduction results in the appearance of males and sexual females. Copulation and fertilization leads to the production of resting eggs, which in Chydoridae are protected by a specially modified molted shell, the ephippium. The ephippium that is detached during molting protects the resting egg during the diapause.
Chitinous chydorid remains, including shells of parthenogenetic (asexually reproducing) females and gamogenetic ephippia, preserve well in lake sediments and can mostly be identified at a species level (Szeroczyńska & Sarmaja-Korjonen, 2007). However, thus far mostly Daphnia ephippia have been utilized (Jeppesen et al., 2003; Johansson et al., 2005), and the analysis of chydorid ephippia has been rare in paleolimnological studies. Sarmaja-Korjonen (1999) found a large abundance of fossil headshields of ephippial Chydorus piger in the sediments of Lake Aitajärvi, in northern Finland, and suggested that high proportions of ephippial headshields are due to long periods of gamogenesis under harsh climatic conditions. Later on, Sarmaja-Korjonen (2003, 2004) presented a new method, ephippium analysis, which uses the relative proportion of fossil ephippia of chydorids in detecting periods of environmental change in lacustrine sediments. In ephippium analysis, the relative proportion of ephippia of all chydorid species constitutes the total chydorid ephippia (TCE) and indicates the relative proportion of sexual reproduction in chydorid communities during the ice-free season. These studies showed that high abundances of chydorid ephippia occurred during the late Pleistocene/early Holocene transect when the climate was harsh in southern Finland because of the close proximity of the Scandinavian Ice Sheet. This finding suggests that sexual reproduction becomes a more important reproductive mode under cold climate conditions. Further evidence on this relationship was recently provided by Bjerring et al. (2009), who found a weak but significant negative relationship between ephippium percentages of chydorids and climate variables (summer and annual mean temperatures) in 54 Pan-European lakes.
Chydorids are hypothesized to change their reproductive mode from asexual to sexual in response to environmental stress and the most common seasonal environmental stress in boreal and subarctic environments is the onset of winter. The original hypothesis for ephippium analysis (Sarmaja-Korjonen, 2004) assumes that in a mild climate, a long open-water (i.e., ice-free) season prevails and the period of parthenogenetic reproduction is long, which results in a high proportion of asexual females (parthenogenetic shells) and a low proportion of sexually reproducing females (ephippia) in lake sediments, i.e., low TCE values. The converse holds, in a cold climate under which the relative length of parthenogenetic reproduction is limited because of a short open-water season, and therefore the relative share of ephippia in sediments is higher. For paleoclimatological purposes, ephippium analysis has been utilized in the reinvestigation of the late-glacial Bølling Sø site in Denmark (Bennike et al., 2004), a study of the 8.2 ka cold event of southern Finland (Sarmaja-Korjonen & Seppä, 2007), detecting the “Little Ice Age” (LIA) in southern Finland (Luoto et al., 2008, 2009), and characterizing Holocene climate trends (Nevalainen et al., in press). In these studies, the TCE value was evidently higher during the known cold climate phases when compared to “normal” or warm periods. The sediment core studies (Sarmaja-Korjonen, 2003; Bennike et al., 2004; Sarmaja-Korjonen & Seppä, 2007; Luoto et al., 2008, 2009; Nevalainen et al., in press) demonstrated that a high TCE may be related to climate conditions. For further evidence, Sarmaja-Korjonen (2007) compared the TCE values of surface sediments of seven lakes situated in different climate zones. As the TCE varied from 2.7 to 4.1% in southern Finland (open-water season ca. 8 months; mean summer temperature ~15.5°C) to 26.2–30% in northern Finland (open-water season ca. 5 months; mean summer temperature ~9.5°C), she concluded that TCE most probably reflects the length of the open-water season.
This study aims to examine the relationship between TCE and environmental variables on a much broader geographical context of 76 lakes in Finland along the latitudinal (60–70°N) climatic gradient. The interdependence of TCE and environmental variables (pH, sampling depth, oxygen, electrical conductivity, and various climatic parameters) were tested statistically to find out the most important factors that affect the proportion of TCE in surface sediments. Furthermore, this study aims to develop a novel model for quantitative climate reconstruction.
Materials and methods
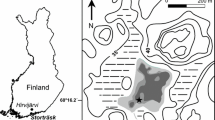
Surface sediment samples from 76 lakes (Fig. 1) were collected during late winter in 2005 using a Limnos gravity corer (Kansanen et al., 1991) through ice. The studied lakes were relatively shallow (<10 m, max sampling depth 7 m) and small (mean ca. 20 ha) with small watersheds. Most of the chosen lakes and the nearby catchments had no known major anthropogenic impact: except for eight lakes in southern Finland. These eight lakes were studied for any influences of environmental stress on gamogenesis (Nevalainen, 2008a; Nevalainen & Sarmaja-Korjonen, 2008a, b). However, it is likely that many of the lakes in southern Finland have had at least minor anthropogenic impacts, which may have influenced trophic state, nutrient level, and food web of the lakes. Figure 2 illustrates the range and trends of selected environmental variables from the 76 lakes along the north–south transect. The altitude of the lakes varied from 11 m a.s.l. in the south to 404 m a.s.l. in the north. The sediment sampling distance from the shoreline was not more than 100 m, and typically below 50 m to include better littoral cladoceran taxa (chydorids) and the samples were taken below 8 m of depth. Luoto (2009) has described the sites in more detail. The topmost 1–2 cm of surface sediment, which represents the most recent years of sedimentation up to the present was collected and stored in plastic bags in a cold room at +4°C. Dissolved oxygen content, pH, and electric conductivity (limnological variables) were measured using a portable (Orion, model 1230) analysator. Unfortunately, due to harsh weather conditions during the sampling period (down to −30°C), the analysator broke. Thus, for the southernmost lakes [mean July temperatures (T July) ranged from 15.6 to 17.0°C] all variables were measured, but for the northernmost lakes only pH and electric conductivity were measured (T July range 11.3–15.2°C). Because of the broken analysator, none of the aforementioned limnological variables in 19 lakes were measured (Fig. 2).
Summary of environmental factors in the 76 Finnish lakes studied (for locations see Fig. 1). Lakes are shown in their order of latitude; northernmost site is at the top of the diagram, and the southernmost site at the bottom of the diagram. Outlier lakes are indicated by a dashed line
Sediment samples for ephippium analysis were prepared by heating and stirring them in 10% potassium hydroxide (KOH) for 20 min and passing the sediment samples through a 44 μm sieve, following the procedure described in Szeroczyńska & Sarmaja-Korjonen (2007). The samples were analyzed for all cladoceran remains, including chydorid ephippia. Identification and nomenclature of subfossil ephippia followed Szeroczyńska & Sarmaja-Korjonen (2007). For ephippium analysis, a minimum of 200 parthenogenetic chydorid shells (+ephippia) were counted. The TCE was expressed as a percentage of the sum of parthenogenetic chydorid shells + ephippia, which determines the proportion of sexual reproduction (ephippia) of all reproduction. Unfortunately, the sum of parthenogenetic shells also included the shells of males because these cannot be distinguished.
Climate data were obtained from the Finnish Meteorological institute (Venäläinen et al., 2005). Mean monthly temperatures for the period 1971–2000 were calculated from daily temperatures that were interpolated onto a 10 * 10 km grid by the kriging method (Henttonen, 1991). The difference between grid mean altitude and site altitude were assessed with a lapse rate of 0.57°C 100 m−1 (Laaksonen, 1976). The length of the open-water season was expressed as the number of days of daily temperatures above 0°C. However, small Finnish lakes need ca. 20 frost-degree days for freezing and ca. 100 degree days for ice cover break up (Korhonen, 2005), and therefore the approximation of the length of the open-water season is most probably slightly biased.
Statistical analyses (Pearson and Spearman correlations, statistical significance, regression, residuals, and descriptive statistics) were performed using the JMP 8 and PASW Statistics 18 programs. Descriptive statistics for species data and stratigraphic diagrams were performed using the C2-program (Juggins, 2007). For the T July reconstruction model, only 73 of the 76 lakes were used. The remaining three lakes were considered as outliers (residuals >2STDres) and excluded; TCE in Lake Pieni Majaslampi (TCE%: 8.9; mean T July: 16.4°C) was affected by recent changes in predation (Nevalainen & Sarmaja-Korjonen, 2008a) and two of the lakes that showed anomalously high TCE values also had a strong odor of monosulphides, and were therefore excluded from model construction. The outlier lakes are indicated in Figs. 1, 2, 3, and 4. The most of the data (TCE, climate variables, conductivity, and oxygen) were not normally distributed (P < 0.01). TCE% was normally distributed after log(10) transformation (Kolmogorov–Smirnov sig. 0.2; Shapiro–Wilk sign 0.43), whereas other variables remained unnormalized. Pearson correlations (r p) between the environmental variables and untransformed TCE% were calculated. For significance testing of correlations, non-parametric Spearman’s correlations (r s) were used.
Chydorid shells and ephippia of selected taxa in the surface sediments of 76 Finnish lakes (for locations, see Fig. 1). Lakes are shown in their order of latitude; the northernmost site is at the top of the diagram, and southernmost site at the bottom of the diagram. Only species of which ephippia was present in at least 20 samples are shown. Abundances are expressed as percentages from chydorid shells + chydorid ephippia. Outlier lakes are indicated by dashed lines
Scatter plots illustrating the relationship between TCE% and selected environmental variables in 76 Finnish lakes. Data points from outlier lakes are indicated as ×. Pearson correlation (r p), number of samples (n), Spearman correlation (r s), and P values are shown. Most of the data are not normally distributed therefore only non-parametric Spearman correlations were tested. The correlations without outliers are shown in parentheses
Results
A list of species found is presented in Table 1. Ephippia of 29 species were found in the surface sediment samples. The most abundant species with ephippia were Alona affinis, A. affinis/quadrangularis, and Alonella nana. Asexual shells of A. nana, Acroperus harpae, A. excisa, and Chydorus sphaericus s.l. were present in most samples. Of some rare species (Monospilus dispar, Camptocercus streletskaye, and Camptocercus fennicus) or species occurring in small numbers (Pseudochydorus globosus), only their asexual shells were found. TCE values ranged from ca. 2 to 5% in southern Finland to more than 25% in northern Finland (Fig. 3). The number of ephippial species in one sample varied between 2 and 10, and the number of ephippial species slightly increased with decreasing temperatures.
The correlations between TCE and the limnological variables (pH, conductivity, oxygen) were weak and statistically insignificant (Fig. 4). TCE showed strong and statistically significant (r s) correlations with all climate variables (Fig. 4) and the best correlation was with T June and T July (r p = 0.83, r s = 0.79, P < 0.001) mean temperatures. The Pearson correlation between TCE and open-water season was −0.74 (r s = −0.79, P < 0.001). The correlation between TCE and temperature sums (sum of degrees of daily temperatures above 5°C) was −0.80 (r s = 0.8 P < 0.001) and between TCE and the growing seasons (number of days when daily temperature is above 5°C) −0.80 (r s = 0.8, P < 0.001). These correlations are illustrated in Fig. 4.
The tested TCE-based models for paleotemperature reconstructions are summarized in Table 2. The TCE values showed the highest Pearson correlation with mean T July, (r p = 0.83, n = 76; r s = 0.79; P < 0.001), and therefore it was selected as the dependent variable for the quantitative TCE-based climate reconstruction model (Figs. 4, 5). Three of the lakes were identified as outliers (residuals >2STD) and were excluded from the analysis. Linear regression (T July = 16.8–0.24 * TCE) showed rate of explanation of 0.78 (P < 0.001) with standard error of 0.73°C (Fig. 5a, solid line). An even better rate of explanation was obtained with non-linear (polynomial, second degree) model (R 2 = 0.81; SE = 0.67°C; Fig. 5a, dashed line). The residuals of linear regression analysis showed decreasing variability with increasing TCE values, except in the two highest TCE values (Fig. 5b). Residuals of the polynomial fit showed similar pattern compared with those of the linear fits, except for the high TCE values (Fig. 5c). However, these data were not normally distributed, and the residuals were heteroscedastic, and therefore requirements of linear regression analysis were not fulfilled. TCE data were transformed by the log10 (skewness −0.026, kurtosis −0.62) to normalize their distribution. The linear regression model with the log-transformed TCE as independent variable (T July = 18.42–4.44log10TCE; Fig. 5d) showed a rate of explanation of 0.76%. The residuals of log-transformed regression (Fig. 5e) had slightly smaller variances in low and high log10TCE values than for the middle values. However, the variation is more even than those of linear or polynomial fits. Figure 5f illustrates the relationship between observed and predicted values with error bars using the linear fit.
Linear and polynomial (a) and linear log-transformed (d) inference models to predict mean July temperatures by using the proportions of TCE in the surface sediments of 73 Finnish lakes. The distribution of residuals between observed and predicted mean July temperatures plotted against TCE are shown for linear (b), polynomial (c), and linear log-transformed models (e). The observed and predicted mean T July and standard error bars for the 73 sites are shown in (f) for the linear model
Discussion
Data estimation
Chydorid remains from surface sediment samples (topmost 1–2 cm) of 76 lakes were analyzed. If the sediment was loose and contained a lot of water, the sampling depth was usually close to 2 cm and conversely, if the sediment was firm, the sampling depth was not more than 1 cm. The sedimentation rate in different lakes can be highly variable and it can also vary within the same lake. As no datings were recorded for the sediments, it remains unknown what period the sampled sediments exactly represented. The samples were taken in 2005, but the meteorological data that were used covers the 1971–2000 climate normal period. The annual temperatures between 1990 and 2005 were 0.2–0.8°C higher than during the normal period used in this study (Venäläinen et al., 2005). Therefore, when interpreting the results it has to be noted that the samples do not strictly represent the same period pertaining to the meteorological data. However, this temporal temperature discrepancy is more likely to generate stochastic error than systematic error. In addition, because TCE is based on the relative proportions and not on the counted individuals, the bias in results would become significant only if very recent (i.e., not more than 15-year-old sediments) had been sampled.
There were more samples obtained from the warmer (long open-water season, low TCE proportions) end of the training set’s temperature gradient and fewer samples at the colder end of the gradient (Fig. 2). Because most of the data were not normally distributed, the basic assumptions behind regression analysis and testing by Pearson’s correlation are not fully achieved. Therefore, the models and statistical conclusions should be treated with caution. Nevertheless, non-parametric tests for correlation corroborate the strong relationship between TCE and climate variables, which are also presented in the scatter plots (Fig. 4).
Relationship between TCE and environmental variables across Finland
The most common chydorid species (A. nana, C. sphaericus s.l., A. excisa, and A. harpae) were present in 72 of the 76 lakes, which suggests that they are well adapted to different environmental conditions. Typically, the ephippia of these species were also present but in exceptionally low numbers in contrast to the relative abundance of their asexual shells (Table 1; Fig. 3).
Based on the present data (Fig. 4), the TCE shows weak and statistically insignificant correlations with the measured non-climatic limnological parameters and high (significant) correlations with climate-related variables (open-water season, growing season, temperature sum, and T July). Therefore, the TCE value appears to be a particularly suitable proxy for climate reconstructions. However, non-climatic parameters can also cause increased intensity in gamogenesis (i.e., sexual reproduction) of some species as indicated by previous monitoring data (Nevalainen, 2008a; Nevalainen & Sarmaja-Korjonen, 2008a), and sedimentary data (Sarmaja-Korjonen, 2003, 2004; Luoto et al., 2008, Nevalainen, 2008b; Nevalainen et al., 2011a). Increased inferred gamogenesis as a consequence of non-climatic environmental stressors, typically manifests in sediment record as high ephippial proportions of only certain chydorid species (Sarmaja-Korjonen, 2003, 2004; Bennike et al., 2004; Luoto et al., 2008; Nevalainen & Sarmaja-Korjonen, 2008b; Nevalainen et al., 2011b).
Chydorus sphaericus s.l., one of the most common chydorid in the present dataset, has very low shares of ephippia (typically <1%, Table 1), and its ephippial shells appear in only less than half of the samples. Ephippia of C. sphaericus s.l. do not show any correlation between climatic variables (cf. Fig. 3). The maximum abundance of ephippia in this species (7.2%) was found in a lake that was considered as an outlier in our dataset (Fig. 3). The lake emitted a strong odor of monosulphides during the late-winter sampling, which suggested that it had deoxygenated near-bottom conditions. This might have acted as a stimulus for intensive sexual reproduction in C. sphaericus s.l. It has been noted that the C. sphaericus-type has a low intensity of gamogenesis during autumn (Nevalainen, 2008a; Nevalainen & Sarmaja-Korjonen, 2008a), which suggests that in some regions it may mostly rely upon parthenogenetic reproduction even in winter. Also, Bennike et al. (2004) suggested that C. sphaericus-type is highly tolerant to severe climatic conditions and reproduces mainly asexually because its ephippia were extremely rare in the late-glacial sequence although the species per se was abundant.
Recently, it was suggested that reproduction mode of A. nana might remain parthenogenetic through winter in southern Finland (Nevalainen & Sarmaja-Korjonen, 2008a). It is known to exist perennially in Norway (Koksvik, 1995) and Estonia (Mäemets, 1961). The increased gamogenesis of A. nana in southern Finland is suggested to be a consequence of high conductivity (Nevalainen & Sarmaja-Korjonen, 2008a) and low oxygen concentrations in winter (Nevalainen et al., 2011b). In the current dataset, ephippia of A. nana showed a slight tendency of increasing ephippial values along with decreasing temperatures (Figs. 2, 3). However, high proportions (8.4 and 8.6%) of A. nana ephippia were found in the outlier lakes that emitted the odor of monosulphides and accordingly the winter oxygen depletion would favor intense gamogenesis for A. nana’s survival in these sites. Sarmaja-Korjonen (2004) found increased proportions of A. nana ephippia in Lake Kaksoislammi’s sediment core as a result of a fish killing episode caused by the rapid fall in pH. However, the present data suggests that the intensity of gamogenesis of A. nana is mainly a response to climate (r TJuly = −0.61, P < 0.001, n = 76) rather than to either oxygen concentration (r = −0.16, P = 0.42, n = 26) or conductivity (r = −0.15, P = 0.28, n = 52). However, in some cases, non-climatic environmental stressors can cause a significant increase in intensity of gamogenesis in A. nana (this study; Sarmaja-Korjonen, 2004; Nevalainen & Sarmaja-Korjonen, 2008a; Nevalainen et al., 2011b).
The identification of increased TCE values in sediment core samples that is caused by non-climatic factors is difficult or even sometimes impossible. In some cases, only one species is affected and in others several species may respond with intensified gamogenesis. Based on the present data and previous studies, we suggest that if only Chydorid sphaericus s.l. or A. nana show a high rate of gamogenesis the climatic interpretation should be treated with caution. Proxies that infer inter alia pH, nutrient status, and oxygen content should be used in parallel with ephippium analysis to find out if a temporary increase in TCE was caused by changes in non-climatic factors.
Relationship between TCE and climate across Finland
All the climate variables correlated strongly (r ~ 0.8) and significantly (P < 0.001) with TCE (Fig. 4). In Finland, climate parameters have a strong south–north gradient (Fig. 2), and therefore a strong intercorrelation between different climate variables is apparent. Consequently, distinguishing the main climatic forcing factors behind changes in TCE values is problematic. The strong intercorrelation between different climatological parameters in the present dataset gave high correlations between TCE and some climatic parameters that may not have a causal relationship to TCE (Figs. 2, 4). The slightly higher Pearson correlation was found between TCE and T July, and not between TCE and open-water season, as was expected (Fig. 4). However, this does not indicate that T July solely interacts with TCE. It can be assumed that the TCE is dependent on at least three climatic factors: (a) summer temperatures, which affect the rate of parthenogenetic reproduction, (b) the length of autumn, which affects the length of the sexual period, and (c) the length of the open-water season, which affects the relative lengths of asexual and sexual reproduction periods. However, the variability caused by non-climatic factors may play an important role for explaining the variance in TCE in some cases.
The intensity of asexual reproduction of chydorids and their relationship to summer temperatures is somewhat unknown, although it has been suggested that the mode is related to water temperatures (Green, 1966; Koksvik, 1995; Nevalainen, 2008a). Typically, the abundance of individuals in chydorid populations increases toward midsummer, decreases during midsummer, and increases again during autumn (Goulden, 1971; Whiteside, 1974; Whiteside et al., 1978; Nevalainen, 2008a). This pattern of change has been suggested to be controlled by competition and/or predation (Goulden, 1971; Adalsteinsson, 1979; Williams, 1983). A study on the abundance of chydorid individuals (asexual females, males, and sexual females) from three lakes in southern Finland (Nevalainen, 2008a) showed high variability in the abundance of asexual and sexual chydorid individuals among the three adjacent lakes suggesting that the chydorids exhibited site-specific patterns in both reproductive modes. However, more ecological monitoring with wide geographical gradients is needed to find out the relationship between water temperatures and chydorid population dynamics.
In Finland, the warmest summer months and the longest open-water season prevail in the southern part of the country where autumn can be relatively warm and last long compared with northern Finland where autumn temperatures fall rapidly (cf. climate normals1971–2000 in Fig. 1). Therefore, the suitable conditions for gamogenesis might last longer in the southern parts of the country and less so in the north.
Samples from southernmost Finland showed evenly distributed TCE values between 1 and 5% (Figs. 3, 4), except for Lake Pieni Majaslampi. This lake is known to have experienced recent acidification-induced changes in predator–prey relationships (Nevalainen & Sarmaja-Korjonen, 2008a). Hypothetically, if the increased intensity of asexual reproduction is caused by high summer temperatures, then the higher summer temperatures will result in a relatively low TCE, as well as causing a longer open-water season. Consequently, if long mild autumns extend the period of sexual reproduction, it should result in higher TCE values. These two mechanisms would then compensate each other in the southernmost Finland. The monitoring of the seven environmentally different lakes in southern Finland during the open-water season in 2005 and 2006 suggest that the length of the gamogenetic reproduction was ca. 2 months (Nevalainen & Sarmaja-Korjonen, 2008b). Moreover, the general patterns of gamogenesis in the studied lakes were synchronous, which suggests falling temperatures and declining photoperiods as main stimuli for the increase of gamogenesis. Nonetheless, there was variability in both the intensity and the duration of gamogenesis among populations in the climatologically similar but environmentally different lakes (Nevalainen & Sarmaja-Korjonen, 2008b). The observed variability in southern Finnish lakes (Fig. 2) in the present dataset (T July > 16°C) can presumably be due to individual non-climatic limnological factors, such as winter oxygen depletion or food-web changes, rather than climatic factors per se. This was also suggested in monitoring studies (Nevalainen, 2008a; Nevalainen & Sarmaja-Korjonen, 2008a, b).
The surface sediment samples from northernmost Finland show high values and ranges of TCE (from 14.4 to 26.9%). In northern Finland all climate parameters; short open-water season, low summer temperatures, and rapidly decreasing temperatures, i.e., short autumn, result in high TCE values. This can be seen in the high TCE values we report (Figs. 3, 4). Sarmaja-Korjonen (2007) suggested that there might be a threshold in northern Finland, above which the role of gamogenesis becomes even more important for the survival of chydorid species. However, the role of non-climatic environmental factors cannot entirely be ruled out. Recently, whitefish (Coregonus peled) has been introduced into many lakes in Finnish Lapland to increase fishing tourism in the area. The recent changes in predator–prey relationships can therefore have lead to an elevated TCE values in some sites. Unfortunately, the location of lakes into which whitefish has been introduced is mostly unknown.
TCE-based inference model for T July
Among the tested models for paleotemperature reconstructions, the polynomial fit (second degree) showed the best rate of explanation, slightly higher than a linear fit, or a log-transformed linear fit (Fig. 5). The main difference between linear and polynomial fit was basically derived from only two samples (highest TCE and lowest observed T July), which can be seen from the residuals (Figs. 5b, c). The raw data does not fully satisfy the assumptions of linear regression as the data used in this study were not normally distributed; warm sites were overrepresented and cold sites underrepresented. Because of the heteroscedasticity of the residuals of linear and polynomial fits (Figs. 5b, c), the standard error might be biased, and therefore it should be treated with caution. Because of the scarcity of data of >20% TCE in this study, applying the polynomial model is doubtful. In the log-transformed model (Fig. 5d), independent variable (TCE) was transformed into a normal distribution, whereas the dependent variable (T July) could not be normalized. The residuals of log-transformed model (Fig. 5e) show a more even variance than in the linear fit. In the linear regression, it is assumed that the dependent variable is normally distributed but that was not the case in our data. Therefore, log-transformed model should also be interpreted with caution.
To find out whether the relationship between T July and TCE is linear or not, more sites should be studied to increase the reliability of the model. Because of non-normal data distribution of TCE, all presented models have some weaknesses that might generate bias in inferences. The simplest adequate model was the linear model, and therefore we currently suggest that the linear model for inferring T July is preferable instead of non-linear model or log-transformed model. The advantage of the linear model is that it uses only one variable, which has a strong interdependence with climate and a weak relationship with other environmental variables.
The maximum inference capability of the T July models is ca. 17°C. Based on the results of this study and that of Bjerring et al. (2009), it can be assumed that the variability in TCE% explained by non-climatic factors becomes more significant when the proportion of climatologically forced gamogenesis is small (~T July is higher than ca. 16°C). However, in the current dataset these samples represent nearly 40% of the total samples, which may cause bias to inference models. The presented models in our study predict underestimated temperatures for observed T July values higher than ca. 16°C (Fig. 5a, d, f). Therefore, we suggest that TCE-based model for T July should not be used in a straightforward manner if TCE is less than 5%. Instead, we suggest that TCE values less than 5% should be taken as an indication for T July of ca. 15.5°C or more.
The performed linear model presented in the current study explains 78% of mean T July variability (Fig. 5) that is comparable with previous Finnish pollen-based (R 2 = 0.85; Heikkilä & Seppä, 2003), chironomid-based (R 2 = 0.78; Luoto, 2009), and Cladocera-based (R 2 = 0.67; Luoto et al., 2011) transfer functions. The chironomid- and Cladocera-based functions have 65 common lakes with lakes in this study. The present TCE-based model underestimates temperatures in the coldest sites (Fig. 5), whereas in chironomid- and Cladocera-based models temperatures of the coldest sites were overestimated (Luoto, 2009; Luoto et al., 2011). The calculated average of predicted T July shows a very high correlation (r p = 0.97; Table 3) with observed T July, which suggests that combining different proxies might be a potential tool for reliable quantitative climate reconstructions. Furthermore, whenever applying the TCE-based model for paleoclimatic inferences, a multiproxy approach should be used to identify and distinguish climate signals from other environmental stresses.
Conclusions
The proportion of TCE in the surface sediments of the 76 Finnish lakes was strongly and significantly correlated between climatic parameters (high TCE in cold climate and vice versa), but weakly and insignificantly correlated with non-climatic environmental parameters. Therefore, TCE has good potential for quantitative paleoclimate reconstructions. However, in some cases, non-climatic parameters can cause intense gamogenesis, which may cause site-specifically high TCE irrespective of climate forcing. The identification of non-climatic stress for chydorid communities obtained from the sediment cores is difficult and to be able to eliminate site-specific biases in climate reconstructions, a multiproxy approach is recommended. The TCE transfer models based on mean July temperatures show favorable performance statistics. However, it remains unanswered, if the relationship between T July and TCE is truly linear or non-linear, because of the scarcity of cold sites in the temperature gradient. Despite this problem, we suggest that ephippium analysis is a valid method to reconstruct past mean July temperatures in cold-temperate regions, such as in high latitudes or altitudes.
References
Adalsteinsson, H., 1979. Seasonal variation and habitat distribution of benthic Crustacea in Lake Mývatn in 1973. Oikos 32: 195–201.
Bennike, O., K. Sarmaja-Korjonen & A. Seppänen, 2004. Reinvestigation of the classic late-glacial Bølling Sø sequence, Denmark: chronology, macrofossils, Cladocera and chydorid ephippia. Journal of Quaternary Science 19: 465–478.
Bjerring, R., E. Becares, S. Declerck, E. M. Gross, L.-A. Hansson, T. Kairesalo, M. Nykänen, A. Halkiewichz, R. Kornijów, J. M. Conde-Porcuna, M. Sereflis, T. Näges, B. Moss, S. L. Amsinck, B. V. Odgaard & E. Jeppesen, 2009. Subfossil Cladocera in relation to contemporary environmental variables in 54 Pan-European lakes. Freshwater Biology 54: 2401–2417.
Frey, D. G., 1982. Contrasting strategies of gamogenesis in northern and southern populations of Cladocera. Ecology 63: 223–241.
Goulden, C. E., 1971. Environmental control of the abundance and distribution of the chydorid cladocera. Limnology and Oceanography 16: 320–331.
Green, J., 1966. Seasonal variation in egg production by cladocera. Journal of Animal Ecology 35: 77–104.
Heikkilä, M. & H. Seppä, 2003. A 11,000 yr palaeotemperature reconstruction from the southern boreal zone in Finland. Quaternary Science Reviews 22: 541–554.
Henttonen, H., 1991. Kriging in interpolating July mean temperatures and precipitation sums. University of Jyväskylä, Department of Statistical Science. Publication No. 12. Jyväskylä: 41 pp.
Jeppesen, E., J. P. Jensen, T. L. Laurisen, S. L. Amsinck, K. Christoffersen, M. Søndergaar & S. Mitchell, 2003. Sub-fossils of cladocerans in the surface sediment of 135 lakes as proxies for community structure of zooplankton, fish abundance and lake temperature. Hydrobiologia 491: 321–330.
Johansson, L. S., S. L. Amsinck, R. Bjerring & E. Jeppesen, 2005. Mid- to late-Holocene land-use change and lake development at Dallund Sø. Denmark: trophic structure inferred from cladoceran subfossils. The Holocene 15: 1143–1151.
Juggins, S., 2007. C2 Version 1.5 user guide. Software for ecological and palaeoecological data analysis and visualisation. Newcastle University, Newcastle upon Tyne: 73 pp.
Kansanen, P. H., T. Jaakkola, S. Kulmala & R. Suutarinen, 1991. Sedimentation and distribution of gamma-emitting radionuclides in bottom sediments of southern Lake Päijänne, Finland, after the Chernobyl accident. Hydrobiologia 222: 121–140.
Kiser, R. V., J. R. Donadlson & P. R. Olson, 1963. The effect of rotenone on zooplankton populations in freshwater lakes. Transactions of the American Fisheries Society 92: 17–24.
Koksvik, J. I., 1995. Seasonal occurrence and diel locomotor activity in littoral cladocera in a mesohumic lake in Norway. Hydrobiologia 307: 193–201.
Korhonen, J., 2005. Jääpeitteen vaihtelut ja trendit Suomen sisävesissä (The variability and trends in ice cover in Finnish lakes). In Viljanen, A. & P. Mäntyniemi (eds), XXII Geofysiikan päivät. Geophysical Society of Finland: 89–93 (in Finnish).
Laaksonen, K., 1976. The dependence of mean air temperatures upon latitude and altitude in Fennoscandia. Annales Academiae Scientarum Fennicae, Series A III 119: 1–19.
Luoto, T. P., 2009. Subfossil Chironomidae (Insecta:Diptera) along a latitudinal gradient in Finland: development of a new temperature inference model. Journal of Quaternary Science 24: 150–158.
Luoto, T. P., L. Nevalainen & K. Sarmaja-Korjonen, 2008. Multiproxy evidence for the ‘Little Ice Age’ from Lake Hampträsk, Southern Finland. Journal of Paleolimnology 40: 1097–1113.
Luoto, T. P., K. Sarmaja-Korjonen, L. Nevalainen & T. Kauppila, 2009. A 700 year record of temperature and nutrient changes in a small eutrophied lake in southern Finland. The Holocene 19: 1063–1072.
Luoto, T. P., L. Nevalainen, S. Kultti & K. Sarmaja-Korjonen, 2011. An evaluation of the influence of water depth and river inflow on quantitative Cladocera-based temperature and lake level inferences in a shallow boreal lake. doi:10.1007/s10750-011-0801-6.
Mäemets, A., 1961. Eesti vesikirbuliste (Cladocera) ökoloogiast ja fenoloogiast. Hüdrobiooloogilised uurimused 2: 108–158. (In Estonian).
Nevalainen, L., 2008a. Partenogenesis and gamogenesis in seasonal succession of chydorids (Crustacea, Chydoridae) in three low-productive lakes as observed with activity traps. Polish Journal of Ecology 56: 85–97.
Nevalainen, L., 2008b. Sexual reproduction in chydorid cladocerans (Anomopoda, Chydoridae) in southern Finland – implication for paleolimnology. PhD thesis. Publications of the Department of Geology D 16:1–54.
Nevalainen, L. & K. Sarmaja-Korjonen, 2008a. Intensity of autumnal gamogenesis in chydorid (Cladocera, Chydoridae) communities in southern Finland, with a focus on Alonella nana (Baird). Aquatic Ecology 42: 151–163.
Nevalainen, L. & K. Sarmaja-Korjonen, 2008b. Timing of sexual reproduction in chydorid cladocerans (Anomopoda, Chydoridae) from nine lakes in southern Finland. Estonian Journal of Ecology 57: 21–36.
Nevalainen, L., T. P. Luoto, S. Levine & M. Manca, 2011a. Paleolimnological evidence for increased sexual reproduction in chydorids (Chydoridae, Cladocera) under environmental stress. Journal of Limnology 70: 255–262.
Nevalainen, L., K. Sarmaja-Korjonen, T. P. Luoto & S. Kultti, 2011b. Does oxygen availability regulate sexual reproduction in local populations of the littoral cladoceran Alonella nana? Hydrobiologia 661: 463–468.
Nevalainen L., T. P. Luoto, S. Kultti & K. Sarmaja-Korjonen, in press. Do subfossil Cladocera and chydorid ephippia disentangle Holocene climate trends? The Holocene.
Sarmaja-Korjonen, K., 1999. Headshields of ephippial Chydorus piger Sars (Cladocera, Chydoridae) females from northern Finnish Lapland: a long period of gamogenesis? Hydrobiologia 390: 11–18.
Sarmaja-Korjonen, K., 2003. Chydorid ephippia as indicators of environmental change – biostratigraphical evidence from two lakes in southern Finland. The Holocene 13: 691–700.
Sarmaja-Korjonen, K., 2004. Chydorid ephippia as indicators of past environmental changes – a new method. Hydrobiologia 526: 129–136.
Sarmaja-Korjonen, K., 2007. Sexual reproduction of chydorids (Amonopoda, Chydoridae) as indicator of climate in recent sediments of Lake Aitajärvi, northern Finnish Lapland. Studia Quaternaria 24: 69–72.
Sarmaja-Korjonen, K. & H. Seppä, 2007. Abrupt and consistent responses of aquatic and terrestrial ecosystems to the 8200 cal. yr cold event: a lacustrine record from Lake Arapisto, Finland. The Holocene 17: 457–467.
Szeroczyńska, K. & K. Sarmaja-Korjonen, 2007. Atlas of subfossil cladosera from central and northern Europe. Friends of the Lower Vistula Society, Poland: 83 pp.
Venäläinen, A., H. Tuomenvirta, P. Pirinen & A. Drebs, 2005. A basic Finnish climate data set 1961–2000 – description and illustrations. Finnish Meteorological Institute reports 2005:5, Helsinki: 27 pp.
Whiteside, M. C., 1974. Chydorid (Cladocera) ecology: seasonal patterns and abundance of populations in Elk Lake, Minnesota. Ecology 55: 538–550.
Whiteside, M. C., J. B. Williams & C. P. White, 1978. Seasonal abundance and pattern of chydorids, Cladocera in mud and vegetative habitats. Ecology 59: 1177–1188.
Williams, J. B., 1983. A study of summer mortality factors for natural population of Chydoridae (Cladocera). Hydrobiologia 107: 131–139.
Acknowledgments
This study was financially supported by the Academy of Finland (Grant no. 1107062, EPHIPPIUM project) and by the Kone Foundation (LOng-term climate impactS on lakE tRophic status, the LOSER project for T.P.L.). For assistance during the fieldwork, we thank Dr. Anu Hakala, Susanna Kihlman and Kimmo Karell. For logistical help during the fieldwork, we thank the personnel of the Kevo Research Station. We thank the two anonymous reviewers for their constructive comments and suggestions that helped us to improve this manuscript. Alisdair Mclean is thanked for checking the language.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: H. Eggermont & K. Martens / Cladocera as indicators of environmental change
Rights and permissions
About this article
Cite this article
Kultti, S., Nevalainen, L., Luoto, T.P. et al. Subfossil chydorid (Cladocera, Chydoridae) ephippia as paleoenvironmental proxies: evidence from boreal and subarctic lakes in Finland. Hydrobiologia 676, 23–37 (2011). https://doi.org/10.1007/s10750-011-0869-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-011-0869-z